Transgene analysis
Contributors to this page: CONABIO, Mexico (Francisca Gasman); IITA, Nigeria (Maria Ayodele); CIMMYT, Mexico (Etienne Duveiller, Huixia Wu, Jonathan Crouch, Monica Mezzalama, Suketoshi Taba, Thomas Payne); Bioversity International, Italy (Ehsan Dulloo); International Seed Federation Representative, Monsanto, Mexico (Juan de la Fuente); CIP, Peru (Marc Ghislain); CIMMYT, Kenya (Stephen Mugo); IRRI, Philippines (Ruaraidh Sackville Hamilton); Danish Seed Health Centre for Developing Countries, Denmark (Jan Torp).
This page shows the latest updates on the development of crop-specific guidelines to maintain the genetic identity of germplasm as received and collected regarding the possible unintentional presence of transgenes.
Here we use the term transgenic organism as synonymous to a living modified organism following the Cartagena Protocol on Biosafety. Transgenic varieties have increasingly taken part of modern agriculture, also in developing countries. Several CGIAR Centres currently have genetically engineered material under development to make available the benefits of modern biotechnology to resource-poor farmers. However, voices of concern have urged us to guarantee the maintenance of the genetic identity of the germplasm collected, received and conserved with particular emphasis on avoidance of the unintentional presence of transgenes.
In this context, it is very important for genebanks to prevent the unintentional introgression of exotic genes, including transgenes, not already present in samples conserved in their collections. Best practices in genebanks should be able to achieve a high degree of probability that an accession maintains its genetic identity over generations.
This activity, aimed at limiting the unintentional presence of exotic genes including transgenes into genebank collections, is in line with agreed CGIAR guiding principles. It includes the creation of a database of field-tested genetically modified (GM) crops worldwide, environmental release status and methods of detection and development of crop-specific approaches to maintain germplasm free from transgenes.
|
Workshop report |
|
Click to open PDF (0.1 MB) |
The information on this page was coordinated by Monica Mezzalama (CIMMYT) and implemented by CGIAR Centres holding crops of relevance [Ruaraidh Hamilton (IRRI, Philippines), Marc Ghislain (CIP, Peru) and Suketoshi Taba (CIMMYT-maize collection)] with the additional participation of external experts (see the full list of contributors at the top of this page). This was implemented in line with the recommendations of the CGIAR Genetic Resources Policy Committee.
A workshop held in Mexico (15-17 August 2007) gathered relevant experts who, on the basis of the “Guiding principles for the development of CGIAR Centres' policies to address the possibility of unintentional presence of transgenes in ex situ collections”, drafted the document “Develop crop-specific guidelines to maintain germplasm genetic identity”.
Guidelines to maintain germplasm genetic identity
The basic operations occurring in a genebank, from the acquisition of a new accession to final storage and distribution, are defined in the figure below. It is understood that for specific crops these operations may differ slightly.
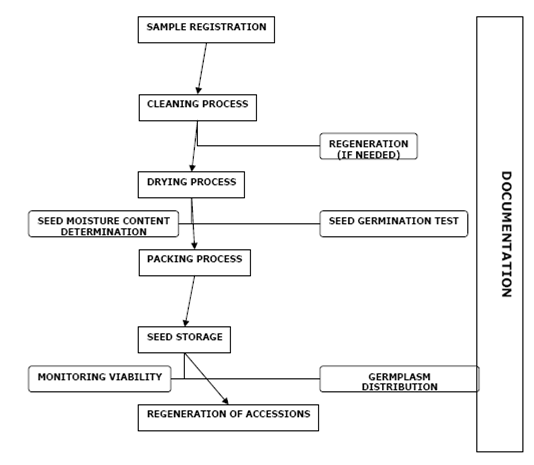
Sequence of procedures in a genebank
Possible risks
A risk regarding transgenes is the occurrence of an unintentional introduction of a transgene or transgenes into a genebank collection during each operation. Indoor/internal (contamination, mixture) and outdoor/external (other released germplasm, experimental germplasm) sources of this risk associated with each operation have been identified and described.
A general schematic description of the guidelines' risk management measures at a non-crop-specific level is shown here.
The establishment of crop-specific regulations was developed in accordance with national and institutional biosafety laws and regulations for maize (prepared by S. Taba), rice (prepared by R. Sackville-Hamilton) and potato (prepared by M. Ghislain) and reviewed and finalized by M. Mezzalama.
Testing procedures
Laboratory testing for GMO detection is recommended as a management measure, depending on the bank operation involved and on the identification and characterization of the source(s) of risk.
Different levels of testing procedures are suggested according to three levels of risk:
- High:
- Refusing introduction of an accession.
- Testing all germplasm to achieve the highest level of confidence.
- Testing using small initial sample size.
- Medium: testing using partial or probabilistic sampling methods to achieve a situation-specific result (depending on crop, source, etc.) at a defined level of confidence.
- Low: no testing, because either no transgenic events are present in the area where the sample was grown or there is no source of risk of gene flow.
Other risk management measures that do not involve testing are described for each operation.
Testing to detect unintentional transgenes
Sample size
The decision on the sample size to be tested should be guided by:
- Statistical indications that can be found in Hernandez-Suarez et al. (2008) and other sources (e.g.,GIPSA, ISTA; see website references).
- The amount of seed available.
- The testing procedures used.
How to test
The decision regarding how to test the material is crop-specific, but it is agreed that DNA-based (rather than protein-based) detection will be essential. It is predicted that in the future, all approved transgenes will have publicly and commercially available markers and detection procedures. Therefore, it is necessary to retrieve and maintain updated information on transgenic constructs and respective detection methods. A website with links to the most accredited external database(s) was prepared. Nevertheless, some of this construct information may be kept confidential and hence may not be public.
When to test
Testing should potentially be carried out at two key stages of genebank operations:
- On new acquisitions, including in situ collections and material originating ex situ, at the point of entry into a genebank.
- On existing accessions before initial use, regeneration and distribution.
Positive results and their publication
A cautious approach should be adopted, respecting the moral integrity of the partners in providing genetic material in good faith, while also recommending full transparency where the provider is aware of the presence of transgenes in the material provided.
Where a positive result is obtained, confidentiality and consultation with the provider in the first instance must be respected in order to obtain all necessary information regarding the material.
Genebank managers should follow the same principles as for the unintentional presence of pathogens and not become a “global GM detection police”.
- Material found positive should usually be destroyed and the provider informed.
- If the material is accepted by the genebank, the center also has a moral obligation to inform the national biosafety authority.
If a sample tests positive: Crisis management responses
- For a new acquisition the following possibilities are suggested:
- Retest to verify.
- Destroy or return to the provider.
- Maintain the sample noting the possible presence of the transgene(s).
- Scrutinize possible sources of contamination.
- Enter into a confidential dialogue with the provider (or donating country authority).
- If the material is accepted into the genebank, national and institutional regulations must be followed, with public (transparent) release of all information.
- For an existing accession the following possibilities are suggested:
- Retest to verify.
- Identify the gene constructs.
- Determine level of presence of the transgenes.
- Inform past recipients, if the accession has previously been distributed.
- Inform the provider (without accusing).
- Inform other genebank holders of the accession (identified through crop registries).
- Maintenance of the sample will depend on the individual bank: as is, or “cleaned” if deemed necessary. Follow guidelines for recognized transgenic accessions.
- Try to minimize any institutional damage.
- For an existing accession that has been distributed and is in a commercialized form, the following suggestions are made:
- Formation of a crisis-management team [(including institutional management, legal advisor, communications expert, biosafety expert, scientist(s)].
- Use of a crisis management reaction matrix (a tool for PR management) to produce a communication strategy.
- Prompt (proactive) timing is essential.
- Issue a global public alert.
- High transparency will result in fewer negative consequences than secrecy. Transparency should also be maintained during ordinary times by cultivating public media that are informed on the types and aims of GM research at CGIAR Centres, as an “educational” activity that may help ameliorate the impact when an undesirable result must be made public.
GMO-free declaration
GMO-“free” statements cannot be issued for technical reasons, except for clonally propagated crops (at high costs). However, transparency is essential and may require a clear statement of procedures used to ensure that material has a low probability of containing transgenes.
Declarations can be issued within stated thresholds and must include stipulations such as “to the best of our knowledge”.
References and further reading
Hernandez-Suarez CM, Montesinos-Lopez O, Crossa J, McLaren G. 2008. Probability models for detecting transgenic plants. Seed Science Research 18:77–89.
Guiding principles for CGIAR Centres' policies to address the possibility of unintentional presence of transgenes in ex situ collections. Available from: http://www.sgrp.cgiar.org/sites/default/files/CGRFA11_Guiding-principles[1].pdf. Date accessed: 22 March 2010.
Booklet of CGIAR Centre Policy Instruments, Guidelines and Statements on Genetic Resources, Biotechnology and Intellectual Property Rights. Statements on Genetic Resources, Biotechnology and Intellectual Property Rights, Version II - Rome, July 2003, 51 pp. Available here.
Interesting websites
The following websites provide descriptions of transgenic events and databases on the events released for each crop in each country; the international biosafety agreement; and technical information on sampling and testing.
Convention on Biological Diversity
Cartagena Protocol on Biosafety
Grain Inspection, Packers and Stockyards Administration (GIPSA): Homepage - Sampling procedures
International Service for the Acquisition of Agri-biotech Applications (ISAAA)
International Seed Testing Association (ISTA)
Sistema de Información de Organismos Vivos Modificados
OECD BioTrack Product Database
Comments
- No comments found





.jpg)
Leave your comments
Post comment as a guest