Bacteria - potato
Contributors to this section: CIP, Lima, Peru (Carols Chuquillanqui, Segundo Fuentes, Ivan Manrique, Giovanna Muller, Willmer Pérez, Reinhard Simon, David Tay, Liliam Gutarra); CIP, Nairobi, Kenya (Ian Barker); FERA, UK (Derek Tomlinson, Julian Smith, David Galsworthy, James Woodhall).
|
Contents: |
Bacterial wilt of potato; Potato brown rot
Scientific name
Ralstonia solanacearum (Smith 1896) Yabuuchi et al. 1996
Significance
EPPO A2 quarantine organism.
Race 3 (biovar 2A) strains of R. solanacearum, which affect mainly potato but occasionally tomato and other solanaceous crops and weeds, are most common in higher elevations of the tropics (up to 3400 masl). At lower elevations, race 1 strains are most prevalent and affect a wide range of crops and weeds. Crops highly susceptible to race 1 (biovars 1, 3 or 4) of R. solanacearum are potato, tobacco, tomato, eggplant, chili, bell pepper, and groundnut (peanut).
Symptoms
Foliage: Wilting (similar to lack of water), stunting and yellowing of the foliage. The browning of vascular bundles may be seen when the cortex is peeled. Characteristic, too, is the initial wilting of only part of the stems of a plant, or even one side of a leaf or stem. If disease development is rapid, the entire plant wilts quickly, without yellowing. Alternatively, the diseased stem can wilt completely and dry up, while the remainder of the plant appears healthy.
Tubers: External symptoms on the tuber are visible at harvest when infection is severe. Bacterial ooze often emerges from the eyes and stem-end attachment of infected tubers. Cut tubers show a pus-like slime coming out of the vascular ring with a slight squeezing or it may exude naturally. In advanced stages of the infection, tubers exhibit brownish discoloration of the vascular ring.
Hosts
Lycopersicon esculentum (tomato), Nicotiana tabacum (tobacco), Solanum melongena (aubergine, eggplant), Solanum tuberosum (potato), Musa spp. (banana), Musa paradisiaca (plantain), Heliconia, Solanum dulcamara, Anthurium spp., Arachis hypogea (groundnut, peanut), Capsicum annuum (bell pepper), Gossypium spp. (cotton), Hevea brasiliensis (rubber), Ipomoea batatas (sweet potato), Manihot esculenta (cassava), Ricinus communis (castor bean), Zingiber officinale (ginger), Solanum cinereum, Solanum nigrum , Galinsoga parviflora, G. ciliata, Polygonum capitata, Portulaca oleracea, Urtica dioica and S. nigrum.
Geographic distribution
Asia, Europe, Africa, North America, Central America, South America, Oceania.
Biology and transmission
Infected seed tubers are the main means of dissemination of R. solanacearum (particularly for race 3 strains). In cool conditions, such as tropical elevations above 2500 m, infected but symptomless plants may harbor the bacterium and transmit it to progeny tubers as latent infection, leading to severe disease outbreaks when grown at warmer locations (French, 1968; Kelman et al., 1994). The pathogen can survive in soil (mostly on plant debris) and in the rooting system and rhizosphere of many hosts (weeds, other host crops, potato volunteers (Moffett et al., 1981; Persley, 1986; Singh, 1995).
Race 3 may be spread in water when infected, S. dulcamara grows in water. The bacterium may subsequently be spread to other hosts when contaminated water is used for irrigation (Elphistone et al., 1998; Wenneker et al., 1999.).
Growing medium accompanying plants, seedlings and micropropagated plants are liable to carry propagules in trade and transport (CABI, 2007).
Detection/indexing method in place at CIP
- In potato tubers: NCM-ELISA (Enzyme Linked Immunoabsorbent Assay on Nitrocellulose Membrane)
-
In potato stems: DAS-ELISA (Double antibody sandwich enzyme-linked inmunoasorbent assay)
Real time PCR for confirming positive results obtained in NCM or DAS ELISA.
Modified Kelman’s medium with Tetrazolium chloride for detection and isolation.
Treatment/control
- In seed certification schemes, no bacterial wilt must be tolerated during the growing season. Stocks of in vitro cultures used for propagation should be from pathogen-free plants and maintained under conditions designed to prevent infection and contamination.
Procedure followed at CIP in case of positive test
- If pathogen is detected and cannot be eradicated, the germplasm must be destroyed. If the germplasm is scarce or unique, maintain it separately under containment so as not to present a risk to other germplasm.
References of protocols at EPPO, NAPPO or other similar organization
NAPPO. 2003. Regional standard for Phytosanitary Measures (RSPM) No.3. Requirements for importation of potatoes into a NAPPO member country. 53 pp.
OEPP/EPPO. 1978. Data sheets on quarantine organisms No. 58. Pseudomonas solanacearum. Bulletin OEPP/EPPO Bulletin 8(2).
OEPP/EPPO. 1990. Quarantine procedures No. 26. Pseudomonas solanacearum, inspection and test methods. Bulletin OEPP/EPPO Bulletin 20:255-262.
OEPP/EPPO. 2004. Ralstonia solanacearum. Bulletin OEPP/EPPO Bulletin 34: 327-329
USDA/APHIS/PPQ.2003. Pest Data Sheet. Ralstonia solanacearum race 3 biovar 2.
References and further reading
CABI. 2007. Crop Protection Compendium [online] Available from URL: www.cabi.org/compendia/cpc/ Commonwealth Agricultural Bureau International (CABI), Wallingford, UK. Date accessed 06 May 2010
Elphinstone JG, Stanford HM, Stead DE. 1998. Detection of Ralstonia solanacearum in potato tubers, Solanum dulcamara, and associated irrigation water. In: Prior P, Allen C, Elphinstone J. (eds.). Bacterial Wilt Disease: Molecular and Ecological Aspects. Berlin, Germany: Springer publishing, 133–139.
French ER, Sequeira L. 1968. Bacterial wilt or moko of plantain in Peru. Fitopatologia, 3:27–38.
Kelman A, Hartman GL, Hayward AC. 1994. Introduction. In: Hayward AC, Hartman GL. (eds.). Bacterial wilt: the Disease and its Causative Agent, Pseudomonas solanacearum. Wallingford, UK: CAB International, 1–7.
Moffett ML, Wood BA, Hayward AC. 1981. Seed and soil: sources of inoculum for the colonization of the foliage of solanaceous hosts by Pseudomonas solanacearum. Annals of Applied Biology, 98(3):403–411
Persley GJ. 1986. Ecology of Pseudomonas solanacearum, the causal agent of bacterial wilt. In: Persley GJ, ed. Proceedings of an International Workshop held at PCARRD, Los Baños, Philippines 8–10 October 1985. ACIAR Proceedings, 13, 71–76.
Samson R, Legendre JB, Christen R, Fischer-Le Saux M, Achouak W, Gardan L. 2005. Transfer of Pectobacterium chrysanthemi(Burkholder et al. 1953) Brenner et al. 1973 and Brenneria paradisiaca to the genus Dickeya gen, nov, as Dickeya chrysanthemicomb. nov. and delineation of four novel species, Dickeya dadantii sp. nov., Dickeya dianthicola sp. nov., Dickeya dieffenbachiaesp. nov. and Dickeya zeae sp. nov. International Journal of Systematic and Evolutionary Microbiology 55:1415–1427
Singh R. 1995. Seed transmission studies with Pseudomonas solanacearum in tomato and eggplant. ICIAR Bacterial Wilt Newsletter, 11:12-13.
Wenneker M, Verdel MSW, Groeneveld RMW, Kempenaar C, Beuningen AR, van Janse JD. 1999. Ralstonia (Pseudomonas) solanacearum race 3 (biovar 2) in surface water and natural weed hosts: First report on stinging nettle (Urtica dioica). European Journal of Plant Pathology, 105(3):307–315.
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the safe movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Specific references on detection:
NCN-ELISA kit for the detection of Ralstonia solanacearum in potato tubers
Priou S, Gutarra L, Aley P. 1999. Highly sensitive detection of Ralstonia solanacearum in latently infected potato tubers by post-enrichment ELISA on nitrocellulose membrane. EPPO Bulletin / Bulletin OEPP 29: 117–125.
Priou S, Salas C, de Mendiburu F, Aley P, Gutarra L. 2001. Assessment of Latent Infection Frequency in Progeny Tubers of Advanced Potato Clones Resistant to Bacterial Wilt: A New Selection Criterion. Potato Research 44: 359–373.
Priou S, Torres R, Villar A, Gutarra L, de Mendiburu F. 2001. Optimization of sample size for the detection of latent infection by Ralstonia solanacearum in potato seed tubers in the highlands of Peru. Potato Research 44: 349–358.
DAS-ELISA kit for the detection of Ralstonia solanacearum in potato stems before harvest of the potato crop
Priou S, Gutarra L, Aley P, de Mendiburu F, Llique R. 2009. Detection of Ralstonia solanacearum (Biovar 2A) in stems of symptomless plants before harvest of the potato crop using post-enrichment DAS-ELISA. Plant Pathology , in press
CIP's detection procedures for Ralstonia solanacearum is available online from URL:
http://www.cipotato.org/potato/pests_diseases/bacterial_wilt/detection_kits.asp Date accessed 06 May 2010
Real-time PCR for detection of Ralstonia solanacearum
Weller SA, Elphinstone JG, Smith NC, Boonham N, Stead DE. 2000a. Detection of Ralstonia solanacearum strains a quantitative, multiplex, real time, fluorogenic PCR (TaqMan) assay. Appl. Environ. Microbiol. 66:2853–2858.
Weller SA, Elphinstone JG, Smith NC, Stead DE. 2000b. Detection of Ralstonia solanacearum from potato tissue by post enrichment TaqMan PCR. EPPO Bull. 30:381–383.
Modified Kelman’s medium with Tetrazolium chloride for detection and isolation of Rs
French ER, Gutarra L, Aley P, Elphinstone J. 1995. Culture media for Pseudomonas solanacearum: isolation, identification and maintenance. Fitopatologia 30, 126–30.
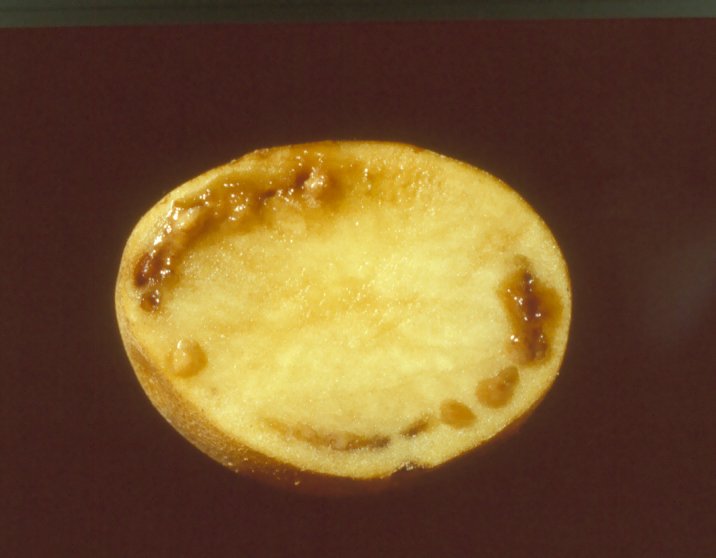 BW on tubers (photo: CIP) |
 Plants with BW symptom (photo: CIP) |
Scientific name
Dickeya chrysanthemi (Burkholder et al. 1953) Samson et al. 2005, comb. nov.
Significance
EPPO A2 quarantine organism.
Erwinia chrysanthemi has been reclassified into six new Dickeya species (Samson et al., 2005). The revised nomenclature of these pathogens has distinguished them from other soft rot erwiniae (including P. atrosepticum and P. carotovorum). Different biovars of D. chrysanthemi have been characterized by biochemical, physiological, serological, and molecular and pathogenicity tests. In potato, different Dickeya species have been found in Europe, viz. D. diathicola, D. dadantii and D. zeae (Wolf, 2007) and in other countries including Australia and Peru (Elphinstone, 2007). Furthermore, Dickeya strains have been found in potatoes grown in Israel, which could not be classified in any of the six new species (van der Wolf, 2007).
Symptoms
Foliage: Wilting and desiccation of foliage. In warmer parts causes increasing incidence of blackleg especially when temperature rises above 25°C, whereas P. atrosepticum typically causes blackleg symptoms under cool wet conditions. The foliar symptoms most commonly associated with D. dianthicola in warm dry growing conditions include brown staining of the vascular tissues and occasionally necrosis and hollowing of the stem, which usually remains green until leaf desiccation is complete (Elphinstone, 2007). Symptoms caused by D. dianthicola under warm dry conditions can be confused with those of the other wilting diseases.
Tubers: Symptoms of soft rot disease on potato tubers are similar whether caused by Dickyea or Pectobacterium spp. In cases of severe infection, progeny tubers are rotten in the soil.
Hosts
The pathogen has been reported worldwide on many hosts as Erwinia chrysanthemi.
Geographic distribution
Asia, Europe, Africa, North America, Central America, South America, Oceania
Biology and transmission
Seed tubers and seed pieces are the primary source of inoculum for Dickeya spp. Factors influencing disease development on potato caused by Dickeya spp. are generally the same as for P. atrosepticum, with the exception of temperature, where a warmer spring and summer favors disease development by Dickeya spp. (Elphinstone, 2007). D. dianthicola biovars 3 and 7 have been reported as more adapted to temperate climates while biovar 3 variant is more adapted to a higher temperature (van der Wolf, 2009; Tsror, 2009; Stead, 1999). Dickeya spp. is more efficient to colonize plant tissue compared to Pectobacterium spp., however it seems to act like biotrophic organism, which needs the host for long-term survival. Dickeya spp. survives poorly in free soil but has been frequently found in surface water, suggesting that it can persist for long periods in water (van der Wolf, 2009; Elphinstone, 2007). The bacteria can invade stems from the mother seed tuber, which can develop blackleg symptoms. The development of black leg depends on time at which the mother tuber rots. Invasions of tubers by bacteria can occur in a number of ways. Immature skins on tubers, wounding and high nitrogen fertilization predispose tubers to soft rot (Wale, 2008).
The bacterium is transmitted in soil and growing medium, and can survive for up to 10 weeks in cattle manure (Lohuis, 1990). Over long distances, and especially across national borders, it is mainly spread by infected vegetative propagating material (CABI, 2007).
Detection/indexing method in place at CIP
- Crystal violet pectato (CVP) medium for detection and isolation of D. chrysanthemi (Pérombelon and Burnet (1991).
- Modified Kelman’s without Tetrazolium chloride (CPG) medium for detection and isolation of D. chrysanthemi (French et al, 1995)
Treatment/control
- In seed certification schemes, no bacterial wilt must be tolerated during the growing season. Stocks of in vitro cultures used for propagation should be from pathogen-free plants and maintained under conditions designed to prevent infection and contamination.
Procedure followed at CIP in case of positive test
- If pathogen is detected and cannot be eradicated, the germplasm must be destroyed. If the germplasm is scarce or unique, maintain it separately under containment so as not to present a risk to other germplasm.
References of protocols at EPPO, NAPPO or other similar organization
NAPPO. 2003. Regional standard for Phytosanitary Measures (RSPM) No.3. Requirements for importation of potatoes into a NAPPO member country. 53 pp.
OEPP/EPPO. 1982. Data sheets on quarantine organisms. No. 53. Erwinia chrysanthemi. Bulletin OEPP/EPPO Bulletin 12 (1).
OEPP/EPPO. 1988. A1 and A2 lists of quarantine pests. Specific quarantine requirements. EPPO Publications Series B No. 92.
OEPP/EPPO. 1990. Specific quarantine requirements. EPPO Technical Documents No. 1008.
References and further reading
CABI. 2007. Crop Protection Compendium [online] Available from URL: www.cabi.org/compendia/cpc/ Commonwealth Agricultural Bureau International (CABI), Wallingford, UK. Date accessed 06 May 2010
Elphinstone J. 2007. Growers’ advice: Erwinia chrysanthemi (Dickeya spp.): What it is, and what you can do. British Potato Council.
Lohuis H. 1990. Does liquid manure spread weeds and bacteria? PSP Pflanzenschutz Praxis, No. 3:28–30.
Samson R, Legendre JB, Christen R, Fischer-Le Saux M, Achouak W, Gardan L. 2005. Transfer of Pectobacterium chrysanthemi (Burkholder et al. 1953) Brenner et al. 1973 and Brenneria paradisiaca to the genus Dickeya gen, nov, as Dickeya chrysanthemi comb. nov. and delineation of four novel species, Dickeya dadantii sp. nov., Dickeya dianthicola sp. nov., Dickeya dieffenbachiae sp. nov. and Dickeya zeae sp. nov. International Journal of Systematic and Evolutionary Microbiology 55:1415–1427
Stead D. 1999. Bacterial diseases of potato: relevance to in vitro potato seed production. Potato Research 42: 449–456
Tsror (Lahkim) L, Erlich O, Lebiush S, Hazanovsky M, Zig U, Slawiak M, Grabe G, van der Wolf JM, van de Haar JJ. 2009. Assessment of recent outbreaks of Dickeya sp. (syn. Erwinia chrysanthemi) slow wilt in potato crops in Israel. European Journal of Plant Pathology 123 (3):311–320
Wale S, Platt HW (Bud), Cattlin N. 2008. Diseases, Pests and Disorders of Potatoes. A Color Handbook. Manson Publishing, London, UK. 176 pp.
van der Wolf JM, Czajkowski R, Velvis H. 2009. Why is Dickeya spp. (syn. Erwinia chrysanthemi) taking over? – The ecology of a blackleg pathogen. In: Symposium KNPV Pests and climate change, 3 December, 2008, Wageningen, The Netherlands
van der Wolf JM, Speksnijder A, Velvis H, van der Harr J, van Doorn J. 2007. Why is Erwinia chrysanthemi (Dickeya sp.) taking over? – The ecology of a blackleg pathogen. In: Hannukkala, A. and M. Segerstedt (eds.). 2007. New and old pathogens of potato in changing climate. Proceedings of the EAPR pathology Section Seminar. 2–6 Th. July 2007, Hattula, Finland.
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the safe movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Specific references on detection:
Pérombelon MCM, Burnett EM. 1991. Two modified crystal violet pectate (CVP) media for detection, isolation and enumeration of soft rot erwinias. Potato Research 34: 79-85.
Modified Kelman’s without Tetrazolium chloride (CPG) medium for detection and isolation of D. chrysanthemi
French ER, Gutarra L, Aley P, Elphinstone J. 1995. Culture media for Pseudomonas solanacearum: isolation, identification and maintenance. Fitopatologia 30, 126-30.
Scientific name
Clavibacter michiganensis subsp. sepedonicus (Spieckermann & Kotthoff) Dye & Kemp
Significance
EPPO A2 quarantine organism.
Symptoms
Foliage: The symptoms develop from mid to late season and usually appear on the lower leaves which are slightly rolled at the margins and are pale green color. As wilting progresses, bars of bright yellow tissue develop between veins. Symptoms may occur on only one or a few stems of a plant and proceed upwards from the lower leaves until the entire stem is wilted. Severely infected plants die prematurely. Potato cultivars vary greatly in their propensity to show symptoms. Symptomless foliage may harbor latent infections especially under cooler conditions. A milky white exudate can be squeezed of stems when cross sectioned at their base.
Tubers: Symptoms are visible at harvest or storage, these begin at stolon end. Discoloration of the vascular tissue is characteristic of this disease; it varies from creamy-yellow to brown areas. When the cut tuber is pressured, creamy odorless ooze may be expressed from the tissue. As the disease progresses, the tissue rounding the vascular ring becomes corky-brown and may develops cavities. Secondary infections increases rotting and tuber disintegrates. Externally, the tubers form diseased plants may appear normal. Sometimes reddish to brown blotches and/or surface cracks are present especially near the eyes. Tuber symptoms may be confused with those caused by the bacterium Ralstonia solanacearum.
Symptomless tubers may harbor latent infections.
Hosts
Lycopersicon esculentum (tomato), Lycopersicon pimpinellifolium (currant tomato), Solanum melongena (aubergine, eggplant), Solanum tuberosum (potato), sugarbeet seed and roots
Geographic distribution
Asia, Europe, Africa, North America, Central America, South America
Biology and transmission
The pathogen overwinters mostly in infected tubers and persists on equipment, machinery and storage (crates, sacks, bags, bins, barrels, etc.) as dried slime (Hooker, 1981, Van der Wolf, 2005). It can overwinter in volunteer plants, in cull piles or in debris from infected crops (Wale et al, 2008). Infection occurs through tuber wounds and invades the xylem vessels and later xylem parenchyma and adjacent tissue and cause separation at the vascular ring (Hooker, 1981). The bacteria migrate from the seed tuber to the stems via the vascular tissue, and subsequently into progeny tubers through the stolons. Disease spread is most frequent when seed tubers are cut and bacteria from infected tubers are transferred onto freshly cut seed surfaces (Wale et al., 2008). Latent pathogen populations in symptomless in vitro plantlets of some potato cultivars can survive several generations (Jeffries, 1998). C. michiganensis subsp. spedenicus is liable to carry in growing medium accompanying plants (CABI, 2007).
Detection/indexing method in place at CIP
- Real time PCR: NCP-88 medium without antibiotics for isolation
Treatment/control
- If pathogen is detected the imported germplasm must be destroyed according Peruvian laws. C. michiganensis subsp. sepedonicus is catalogued as A1 quarantine pathogen for Peru.
Procedure followed at CIP in case of positive test
- If pathogen is detected and cannot be eradicated, the germplasm must be destroyed. If the germplasm is scarce or unique, maintain it separately under containment so as not to present a risk to other germplasm.
References of protocols at EPPO, NAPPO or other similar organization
NAPPO. 2003. Regional standard for Phytosanitary Measures (RSPM) No.3. Requirements for importation of potatoes into a NAPPO member country. 53 pp.
OEPP/EPPO. 2004. Clavibacter michiganensis subsp. sepedonicus. Bulletin OEPP/EPPO Bulletin 34: 323–325
OEPP/EPPO. 2006. Clavibacter michiganensis subsp. sepedonicus. Bulletin OEPP/EPPO Bulletin 36: 99–109
References and further reading
CABI. 2007. Crop Protection Compendium [online] Available from URL: www.cabi.org/compendia/cpc/ Commonwealth Agricultural Bureau International (CABI), Wallingford, UK. Date accessed 06 May 2010
EPPO/CABI. 1992. Pseudomonas solanacearum. In: Smith IM, McNamara DG, Scott PR, Harris KM (E KM, eds.) Quarantine Pests for Europe. Wallingford, UK: CAB International.
Franc GD. 1999. Persistence and latency of Clavibacter michiganensis subsp. sepedonicus in field-grown seed potatoes. Plant Disease, 83(3):247-250.
Hooker WJ. (ed.). 1981. Compendium of Potato Diseases. American Phytopathological Society. St. Paul, Minnesota, USA. 125 pp.
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the Safe Movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
van der Wolf JM, Elphinstone JG, Stead DE, Metzler M, Müller P, Hukkanen A, Karjalainen R. 2005. Epidemiology of Clavibacter michiganensis subsp. sepedonicus in relation to control of bacterial ring rot. Report 95. Plant Research International. Wageningen, The Netherlands. 38 pp.
Wale S, Platt HW (Bud), Cattlin N. 2008. Diseases, Pests and Disorders of Potatoes. A Color Handbook. Manson Publishing, London, UK. 176 pp.
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Specific references on detection:
Real-time PCR for detection of Clavibacter michiganensis subsp. Sepedonicus
Shaad W, Berthier-Shaad Y, Sechler A, Knorr D. 1999. Detection of Clavibacter michiganensis subsp. Sepedonicus in potato tubers by Bio PCR and automated real-time fluorescence detection system. Plant Disease 83, 1095–1100.
NCP-88 medium without antibiotics for isolation
OEPP/EPPO. 2006. Clavibacter michiganensis subsp. sepedonicus. Bulletin OEPP/EPPO Bulletin 36: 99–109
Comments
- No comments found





Leave your comments
Post comment as a guest