Fungi - wheat
Contributors to this page: CIMMYT, Mexico (Etienne Duveiller, Monica Mezzalama, Eloise Phipps, Thomas Payne, Jesper Norgaard), Independent consultant (Jesse Dubin).
|
Spores of Alternaria triticina (photo: CIMMYT)
Note that the chlorotic borders of the lesions |
Scientific name
Alternaria triticina Prasada & Prabhu.
Importance
Reported in Argentina, India, Italy, Mexico, North Africa. High phytosanitary importance.
Significance
Alternaria leaf blight primarily occurs in South Asia, where Alternaria triticina was first identified and considered to be causing foliar blight. Many reports of occurrence of this pathogen are not supported by proper pathogenicity tests, and therefore secondary colonization by saprotophic Alternaria spp. is often mistakenly considered responsible for foliar blight actually caused by Cochliobolus sativus. Although the disease was reported to be severe in the 1960s and 1970s in warmer wheat-growing areas in South Asia and caused major losses when susceptible cultivars were grown, it is likely that yield losses are now rare and much lower than is thought. Old durum wheat cultivars are susceptible (e.g., Bansi). However, recent research indicates that wheat genotypes from South Asia and Mexico are resistant to A. triticina, suggesting that it is not an important pathogen on modern wheat cultivars.
Symptoms
Initial symptoms of Alternaria leaf blight consist of small (< 1 mm), oval, yellow lesions, irregularly scattered on the leaves. As the lesions enlarge, they appear irregular and dark brown to grey, surrounded by a bright yellow margin.
At a later stage, the lesions coalesce, covering large areas of the leaf and sometimes causing plant death (Prabhu and Prasada 1966). Infection usually starts on the lower leaves, but symptoms can be found on all plant parts. Lesions are difficult to distinguish from spot blotch in the absence of C. sativus conidia. Alternaria triticina has often been reported as part of the foliar blight complex.
Hosts
Bread wheat and durum wheat. Several grasses have been reported as primary hosts, but Prabhu and Prasada (1966) were unable to show its virulence on eight Poaceae species, including barley (Hordeum vulgare) and oats (Avena sativa).
Geographic distribution
The disease is common in the eastern and central areas of the Asian subcontinent. It has been reported in Argentina, Italy, Mexico, and North Africa. Reports not supported by pathogenicity tests should be considered with caution and require confirmation.
Biology and transmission
The fungus survives as conidia on seed or as mycelia within seed. Sporulation on lower leaves provides inoculum that can be dispersed by wind, leading to secondary spread of the disease. Seedborne inoculum often results in spike infections late in the crop cycle. High humidity or irrigation, as well as warmer temperatures (20 to 25°C), favor infection and disease development. Like other Alternaria species, A. triticina produces toxic metabolites. The identification of Alternaria species based on morphological criteria applied to colonies and spores remains a challenging task (Maraite et al. 1998). Nevertheless, the presence on 7-day-old PCA of Simmons and Roberts’ sporulation pattern 6 and a restricted number of conidia (two to three) per sporulation unit, associated with primary conidia and long (over 7 μm) apical secondary conidiophores, are useful criteria to differentiate A. triticina isolates from some of the other nonpathogenic Alternaria species (Mercado-Vergnes et al. 2006).
Detection/indexing methods used
- At CIMMYT: Freezing blotter test.
- At ICARDA: Freezing blotter test
Treatment/control
- Many fungicides control external but not internal inoculum. However, treating seed with iprodione and thiram is claimed to provide effective treatment. An alternative method is soaking seed in water at 52 to 54°C for 10 minutes.
Procedures followed at the centers in case of positive test
- At CIMMYT: The seed lot is destroyed (the pathogen is quarantined in Mexico).
- At ICARDA: Not applicable
References and further reading
Adame Beltran E, Diaz Franco A.1997. Tizón de la hoja y rendimiento de trigo asociados a variedad, densidad y fecha de siembra. Revista Mexicana de Fitopatología 15: 31-36.
Agarwal VK, Chahal SS, Mathur SB. 1993. Alternaria leaf blight. In: Mathur SB, Cunfer BM. (eds.), Seed-borne Diseases and Seed Health Testing of Wheat. Copenhagen: Danish Government Institute of Seed Pathology for Developing Countries. pp. 9-11.
Anahosur KH. 1978. Alternaria triticina. CMI Descriptions of Pathogenic Fungi and Bacteria No. 583. Slough, UK: Commonwealth Agricultural Bureaux.
Casulli F. 1990. Epidemiologia delle principali malattie flogliari del frumento duro, in Puglia e Basilicata. Phytopathologia Mediterranea 29: 151-8.
Chaurasia S, Chand R, Joshi AK. 2000. Relative dominance of Alternaria triticina Pras. et Prab. and Bipolaris sorokiniana (Sacc.) Shoemaker in different growth stages of wheat (T. aestivum L.). Journal of Plant Diseases and Protection 107: 176–81.
CIMMYT. Wheat Doctor information sheet: Alternaria leaf blight. [online] Available from URL: http://wheatdoctor.cimmyt.org/. Date accessed 07 April 2010
Diekmann M, Putter CAJ. (eds.). 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14: Small Grain Temperate Cereals. Rome: Food and Agriculture Organization of the United Nations (FAO) and International Plant Genetic Resources Institute (IPGRI). pp. 22-23.
Maraite H, Di Zinno T, Longree V. Daumerie, Duveiller E. 1998. Fungi associated with foliar blight of wheat in warm areas. In: Duveiller E, Dubin HJ, Reeves , McNab A. (eds.), Helminthosporium Blights of Wheat: Spot Blotch and Tan Spot. Proceedings of the International Workshop on Helminthosporium Diseases of Wheat: Spot Blotch and Tan Spot, El Batán, Feb 9-14, 1997. Mexico D.F.: CIMMYT. pp. 293–300.
Mercado-Vergnes D, Renard ME, Duveiller E, Maraite H. 2006. Identification of Alternaria spp. on wheat by pathogenicity assays and sequencing. Plant Pathology 55: 485-493.
Perelló AE, Sisterna MN. 2006. Leaf blight of wheat caused by Alternaria triticina in Argentina. New disease report. Plant Pathology 55: 303.
Prabhu AS, Prasada R. 1966. Pathological and epidemiological studies on leaf blight of wheat caused by Alternaria triticina. Indian Phytopathology 19: 95–112.
Simmons EG. 1992. Alternaria taxonomy: current status, view-point, challenge. In J. Chelkowski and A. Visconti (eds.), Alternaria. Biology, Plant Diseases and Metabolites. Amsterdam: Elsevier. pp. 1-35.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 27.
|
The ergot bodies (sclerotia) can reach 20 mm
Note as the spike matures, kernels of infected
|
Scientific name
Claviceps purpurea (Fr.) Tul.
Other scientific names
Sphacelia segetum Lév. (anamorph)
Importance
High phytosanitary importance.
Significance
Moderate economic importance. Yield losses tend to be small, but losses due to reduced grain quality can be significant and occur worldwide. Ergot alkaloids cause ergotism (resulting in illness and death) in humans and animals.
Symptoms
At flowering, infected florets produce a yellowish, sticky, sweet exudate (containing conidia) that is visible on the glumes. As the spike matures, kernels of infected florets are replaced by brown to purplish black fungal structures (sclerotia or ergot bodies). These ergot bodies can reach 20 mm in length. The whole seed is transformed into a perennation and infection structure as a result of infection.
Hosts
Ergot is found in all small grain cereal crops, especially if sterility occurs for some reason (e.g., frost). Sterile florets tend to open and thus become more liable to infection.
Geographic distribution
Worldwide. The disease is more prevalent in cool, humid climates.
Biology and transmission
Primary infections originate from ascospores in fruiting bodies produced by sclerotia from the previous year’s crop. Ascospores infect the florets, which then produce the sticky exudate containing conidia. Insects are attracted to the sweet exudate, and carry conidia to healthy florets in the same spike or to adjacent spikes. Rainy or humid weather favors the production of exudate and spores. An ergot body develops in each infected floret; these fungal structures can survive in the soil from one season to the next, and under dry condition can remain viable for many years. Sclerotia require cold temperatures before they can germinate.
Detection/indexing methods used
- At CIMMYT: Visual inspection of seed.
- At ICARDA: Visual inspection
Treatment/control
- None
Procedures followed at the centers in case of positive test
- At CIMMYT: Seed is discarded or cleaned.
- At ICARDA: Quarantine pathogen, seed lot is destroyed
References and further reading
CIMMYT. Wheat Doctor information sheet: Ergot. [online] Available from URL: http://wheatdoctor.cimmyt.org/. Date accessed 07 April 2010
Prescott JM, Burnett PA, Saari EE, Ransom J, Bowman J, de Milliano W, Singh RP, Bekele G. 1986. Wheat Diseases and Pests: A Guide for Field Identification. Mexico, D.F.: CIMMYT. pp. 34-35. [online] Available from URL: http://www.cimmyt.org/. Date accessed 07 April 2010
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 55.
Wiese MV. 1987. Compendium of wheat diseases. 2nd edn. St Paul, MN: APS Press. pp. 14-15.
Spot blotch; common root rot
|
Spot blotch: Here you see that as lesions mature
Spot blotch: Here you see that as lesions mature
Symptoms of Common Root Rot on the wheat
|
Scientific name
Cochliobolus sativus (Ito & Kurib.) Drechsler ex Dastur (anamorph. Bipolaris sorokiniana (Sacc.) Shoem.)
Other scientific names
Helminthosporium sativum
Importance
A common seedborne pathogen with minor phytosanitary importance.
Significance
Economically important in warmer non-traditional wheat-growing areas, and the major leaf blight pathogen in such areas. Wheat cultivars in South Asia have shown yield losses of more than 20% (Sharma and Duveiller 2004).
A common root pathogen in dryland wheat-growing areas.
Symptoms
Cochliobolus sativus causes seedling blight, node cankers and spot blotch on leaves.
Depending on the environmental conditions, it can also cause common root rot, characterized by brown spotting and more general necrosis on the sub-crown internodes.
Early leaf lesions resulting in spot blotch, which are the most frequently-observed symptom in warmer, irrigated wheat-growing areas, are characterized by small, dark brown lesions, 1–2 mm long, without a chlorotic margin. In susceptible genotypes, these lesions extend quickly to form oval to elongated, light brown to dark brown blotches that reach several centimeters in length before coalescing and causing the death of the leaf. Abundant production of conidia can be observed on old lesions, and chlorotic streaks are sometimes seen spreading from the borders of lesions as a result of toxin production.
Although different lesion types have been reported in relation to host resistance, lesions in the field progress and coalesce in much the same way, irrespective of genotype
C. sativus produces several sesquiterpenoid toxins. The most important is helminthosporol (HL). This metabolite and its dialdehyde analogue, helminthosporal, are the substances considered to be responsible for inducing spot blotch symptoms.
Hosts
Wheat, barley, rye, oat, triticale and many grasses.
Geographic distribution
Cosmopolitan. Common root rot is mainly found in dryland areas whereas spot blotch occurs in warmer and humid environments. However, there are areas where both diseases can be found.
Spot blotch is important in Bangladesh, Bolivia, Brazil, Paraguay, Zambia, Eastern India, and Nepal.
Common root rot is severe in Australia, parts of Brazil, parts of Kazakhstan, Canada and the USA.
Biology and transmission
The pathogen is seedborne. It survives in soil and also overwinters on crop residues.
The fungus is hemi-biotrophic and heterothallic.
The teleomorph has only been reported under natural conditions in Zambia and is never found in other areas where spot blotch prevails. It develops at 16-24°C in vitro with an optimum at 20°C, whereas the anamorph Bipolaris sorokiniana grows easily in natural conditions or on general synthetic media between 4 and 36°C. B. sorokiniana conidiophores develop as erect, unbranched, single or clustered structures that are 100-150 μm x 6-8 μm in size. The conidia are olive-brown, oblong, tapered towards the ends and have a prominent basal scar. They measure 15-20 μm x 60-120 μm and have three to nine thick-walled septa.
Detection/indexing methods
- At CIMMYT: Freezing blotter test.
- At ICARDA: Freezing blotter test
Treatment/control
- Fungicides such as captan, guazatine plus iprodione, thiram, and triadimephon have been reported to control the pathogen in seed.
- Vitavax-200 improves germination and crop establishment but does not consistently reduce seedling infections (Sharma-Poudyal et al. 2005).
Procedures followed at the centers in case of positive test
- At CIMMYT: Seed treatment with carboxin and captan.
- At ICARDA: Seed treatment
References and further reading
CIMMYT. Wheat Doctor information sheet: Spot blotch. [online] Available from URL: http://wheatdoctor.cimmyt.org.
Extended information sheet: Spot blotch. [online] Available fro URL: http://wheatdoctor.cimmyt.org. Date accessed 07 April 2010
CIMMYT. Wheat Doctor information sheet: Common root rot. [online] Available from URL: http://wheatdoctor.cimmyt.org/ Date accessed 07 April 2010
Diekmann M, Putter CAJ. (eds.). 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14: Small Grain Temperate Cereals. Rome: Food and Agriculture Organization of the United Nations (FAO) and International Plant Genetic Resources Institute (IPGRI). pp. 38-39.
Duveiller E, Dubin HJ. 2002. Helminthosporium leaf blights: spot blotch and tan spot. In: Curtis BC, Rajaram S, Gómez Macpherson H. (eds.), Bread Wheat: Improvement and Production. FAO Plant Production and Protection Series No. 30. Rome: Food and Agriculture Organization of the United Nations (FAO). [online] Available from URL: http://www.fao.org/. Date accessed 07 April 2010
Duveiller E, Dubin HJ, Reeves J, McNab A. (eds.). 1998. Helminthosporium Blights of Wheat: Spot Blotch and Tan Spot. Proceedings of the International Workshop on Helminthosporium Diseases of Wheat: Spot Blotch and Tan Spot, El Batán, Feb 9-14, 1997. Mexico D.F.: CIMMYT. 376pp.
Mehta YR. 1993. Spot blotch. In: Mathur SB, Cunfer BM. (eds.), Seed-borne Diseases and Seed Health Testing of Wheat. Copenhagen: Danish Government Institute of Seed Pathology for Developing Countries. pp. 105-112.
Mercado-Vergnes D, Renard ME, Duveiller E, Maraite H. 2006. Effect of growth stage on host sensitivity to helminthosporol toxin and susceptibility to Cochliobolus sativus causing spot blotch on wheat. Physiological and Molecular Plant Pathology 68: 14-21.
Prescott JM, Burnett PA, Saari EE, Ransom J, Bowman J, de Milliano W, Singh RP, Bekele G. 1986. Wheat Diseases and Pests: A Guide for Field Identification. Mexico, D.F.: CIMMYT. pp. 24-25 and 48-49. [online] Available from URL: http://www.cimmyt.org/. Date accessed 07 April 2010
Reis EM. 1991. Integrated disease management – the changing concepts of controlling head blight and spot blotch. In: Saunders DA. (ed.), Wheat for the Nontraditional Warm Areas: A Proceedings of the International Conference, July 29–August 3, 1990, Foz do Iguacu, Brazil. Mexico, D.F.: CIMMYT. pp. 165-177.
Sharma RC, Duveiller E. 2004. Effect of Helminthosporium leaf blight on the performance of timely and late seeded wheat under optimal and stressed levels of soil fertility and soil moisture. Field Crops Research 89: 205-218.
Sharma-Poudyal D, Duveiller E, Sharma RC. 2005. Effects of seed treatment and foliar fungicides on Helminthosporium leaf blight and performance of wheat in warmer growing conditions. Journal of Phytopathology 153: 401-408.
Sivanesan A. 1987. Graminicolous species of Bipolaris, Curvularia, Drechslera, Exserohilum and their teleomorphs. Mycological Papers No. 158. Wallingford, UK: CAB International Mycological Institute. pp. 62-65.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 27.
Wiese MV. 1987. Compendium of wheat diseases. 2nd edn. St Paul, MN: APS Press. pp. 53-55.
Leaf spot/blotch; foot rot
Scientific name
Cochliobolus spicifer Nelson (anamorph Bipolaris spicifera (Bainier Subram.))
Other scientific names
Curvularia spicifera, Helminthosporium spiciferum, Helminthosporium tetramera
Importance
Negligible
Significance
None
Symptoms
Leaf blotch.
Foot rot in winter wheat.
Hosts
Wheat, maize, barley.
Geographic distribution
Worldwide.
Biology and transmission
Seedborne.
Detection/indexing methods used
- At CIMMYT: Freezing blotter test..
- At ICARDA: Not applicable
Treatment/control
- No specific treatment.
Procedures followed at the centers in case of positive test
- At CIMMYT: Seed treatment with carboxin and captan.
- At ICARDA: Seed treatment
References and further reading
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 17.
Zillinsky FJ. 1981. Common Diseases of Small Grain Cereals: A Guide to Identification. Mexico, D.F.: CIMMYT. p. 23.
Scientific name
Cochliobolus victoriae Nelson (anamorph Drechslera victoriae (Meehan & Murphy) Subram. & Jain)
Other scientific names
Bipolaris victoriae, Helminthosporium victoriae
Importance
Negligible; restricted to oat. However it is quite often found on wheat seed.
Significance
Negligible in wheat.
Symptoms
Leaf spot on oat
Hosts
Oat.
Geographic distribution
North America, Australia, Europe (Scotland, Switzerland, The Netherlands, Ireland), Argentina, Bolivia, Brazil, India, Malaysia, Saudi Arabia, Zambia, Zimbabwe.
Biology and transmission
Seeds carrying mycelium and dead plant tissue infected with pseudothecia act as sources of inoculum.
Detection/indexing methods used
- At CIMMYT: Freezing blotter test.
- At ICARDA: Not applicable
Treatment/control
- No specific treatment
Procedures followed at the centers in case of positive test
- At CIMMYT: Seed treatment with carboxin and captan.
- At ICARDA: Not applicable
References and further reading
Diekmann M, Putter CAJ. (eds.). 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14: Small Grain Temperate Cereals. Rome: Food and Agriculture Organization of the United Nations (FAO) and International Plant Genetic Resources Institute (IPGRI). p. 42.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. pp. 18 and 70.
Zillinsky FJ. 1981. Common Diseases of Small Grain Cereals: A Guide to Identification. Mexico, D.F.: CIMMYT. p. 22.
Fusarium head blight (FHB), head blight, scab
crown rot, foot rot, seedling blight, dryland root rot
|
Scab (also known as Head blight) (Fusarium
Symptoms of Crown Root Rot on the wheat
|
Scientific name
Gibberella zeae (Schwein.) Petch, (anamorph Fusarium graminearum Schwabe) and Gibberella avenacea Cook (anamorph Fusarium avenaceum (Fr.) Sacc.)
Importance
High. Seedborne. Present in Mexico
Significance
G. zeae is the most important and the most studied of the species that cause FHB, which can be caused by more than 15 species depending on environment and geographic location. It causes yield losses and shriveled grains, and most importantly produces a trichothecene mycotoxin, deoxynivalenol (DON).
FHB is one of the most important wheat diseases after the rusts.
G. zeae predominates in moderate to warmer climate, especially where maize and rice are grown, whereas G. avenacea is prevalent in cool climate areas where pasture grasses and legumes are grown. G. avenacea has proved to be mildly or moderately virulent and is less important than F. graminearum. F. culmorum is adapted to intermediate temperatures, while F. poae appears in drier environments.
G. zeae is the only Gibberella/Fusarium species causing significant seedling losses in countries with maritime climates.
Symptoms
Head scab causes premature bleaching or death of individual spikelets, parts of the ear or the entire ear. Infected spikelets are often sterile. Infection of the rachis can cause the whole head to become bleached. In severely-diseased heads and under humid weather conditions, a dark brown discoloration may extend from the diseased spikelets onto the peduncle. White mycelium and pink to orange sporodochia can often be seen at the edges of the glumes or at the base of the spikelet. As the head matures, small, black perithecia may become visible on the glumes. Seeds from diseased heads may show a pink discoloration and be small and shrivelled. Known as ‘tombstones’, diseased kernels can be removed by grading or winnowing with a fan. When diseased seed is planted, seedling blight may develop and seedlings die prior to or after emergence. Less severe infection may result in disease of the coleoptile, subcrown internode, leaf sheaths and primary roots.
In dry environments the disease may result in the development of foot rot, or crown and root rot of adult plants, which show darkening or browning of the root, crown, and basal culm tissues. Individual plants or groups of plants may lodge. White spikes are often visible just prior to normal maturity.
Hosts
Wheat, oat, barley, rye, triticale, maize and numerous grasses.
Geographic distribution
Cosmopolitan.
Biology and transmission
Host debris on or near the soil surface is the principal site of survival. Scab does not arise from seedborne inoculum, but from either macroconidia or ascospores produced on plant debris and dispersed by air currents. It may also develop from chlamydospores or hyphal fragments. Infection occurs principally between the anthesis and soft dough stages. It is favored by continuous wetness of the spikelet (for at least 24 hours) and temperatures of 20-30°C.
Wheat grown after maize is a risk factor, particularly under zero tillage.
Detection/indexing methods
At CIMMYT:
- Freezing blotter test.
- Using the freezing blotter method, fine, white mycelium can be seen on the seed surface with shiny sporodochia of macroconidia. Sporodochia range in color from pale to dull orange (whereas those of F. culmorum are dull orange to whitish pink and those of G. avenacea are bright orange to red).
- The exact identification of the species is difficult and requires very careful observation under the microscope. Confirmation using molecular tools may be needed.
- Germination test on sterile soil.
At ICARDA: Freezing blotter test, Agar test
Treatment/control
- Seed treatment with appropriate fungicides.
Procedures followed at the centers in case of positive test
- At CIMMYT: Seed treatment for shipment with carboxin and captan on seed for export..
- At ICARDA: Seed treatment
References and further reading
Bergstrom GC. 1993. Scab (Fusarium head blight). In S.B. Mathur and B.M. Cunfer (eds.), Seed-borne Diseases and Seed Health Testing of Wheat. Copenhagen: Danish Government Institute of Seed Pathology for Developing Countries. pp. 83-93.
CIMMYT. Wheat Doctor information sheet: Scab (head blight). [online] Available fro URL: http://wheatdoctor.cimmyt.org/. Extended information sheet: Fusarium head blight. [online] Available from URL: http://wheatdoctor.cimmyt.org. Date accessed 07 April 2010
CIMMYT. Wheat Doctor information sheet: Common root rot. [online] Available from URl: http://wheatdoctor.cimmyt.org/. Date accessed 07 April 2010
Diekmann M, Putter CAJ. (eds.). 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14: Small Grain Temperate Cereals. Rome: Food and Agriculture Organization of the United Nations (FAO) and International Plant Genetic Resources Institute (IPGRI). pp. 31-33.
Leslie J, Summerell BA. 2006. The Fusarium Laboratory Manual. Ames, IA: Blackwell Publishing.388 pp.
Prescott JM, Burnett PA, Saari EE, Ransom J, Bowman J, de Milliano W, Singh RP, Bekele G. 1986. Wheat Diseases and Pests: A Guide for Field Identification. Mexico, D.F.: CIMMYT. pp. 32-33 and 48-49. [online] Available from URL: http://www.cimmyt.org/. Date accessed 07 April 2010
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 1.
Wiese MV. 1987. Compendium of Wheat Diseases. 2nd edn. St. Paul, MN: APS Press. pp. 16-18.
|
Leaves in crop showing Cephalosporium leaf stripe (photo: Cereal Disease Encyclopedia) |
Scientific name
Hymenula cerealis (Ellis & Everh.) (anamorph Cephalosporium gramineum Nisikado & Ikata)
Other scientific names
Phialophora cerealis
Importance
High. This is a soilborne pathogen quarantined in Mexico. Infected seed can provide an important source of inoculum for introducing the pathogen and initiating epidemics in areas where the pathogen did not previously occur (Murray, 2006).
Significance
Cephalosporium stripe is a chronic yield-reducing disease that occurs every year in the Pacific Northwest region of the United States. Bruehl (1957) concluded that the pathogen was seed-transmitted, but that the rate was too low to produce these epidemics.
Yield losses can reach up to 80-100% in some fields.
Symptoms
Affected plants are usually randomly scattered through the crop. The disease causes yellow stripes and brown streaks on leaf sheaths and blades. Affected tillers usually have a single distinct bright yellow stripe on each leaf which extends onto the leaf sheath; not all tillers on a plant show symptoms. May cause premature death.
Hosts
Wheat, triticale, oat, barley and several grass species. The disease mainly occurs on winter wheat but spring wheat is also susceptible.
Geographic distribution
Japan, parts of the USA, the UK.
Biology and transmission
The fungus is soilborne and is the only true vascular disease in wheat, colonizing the plant through the xylem. It has been detected on seed but transmission to seedling from infected seed was only recently confirmed (Murray, 2006). The seed-infection rate is low.
The fungus produces sporodochia up to 1mm long on wheat straw. These sporodochia produce conidia.
The disease is favored by wet soils, fluctuating winter temperatures, and repeated cropping of susceptible cereals or grasses in the same field. The fungus survives between seasons as mycelium and conidia on crop residue. It can persist up to 8 cm from the soil surface.
Detection/indexing methods used
- At CIMMYT: Freezing blotter test.
- At ICARDA: Freezing blotter test
Treatment/control
- No seed treatment reported.
- Clean cultivation is required to prevent the multiplication of the pathogen on weeds. Rotations and use of non-host crops for at least two years are recommended following a disease outbreak. Where this is not possible, removing the crop residue and plowing deeper than 8 cm should be considered.
Procedures followed at the centers in case of positive test
- At CIMMYT: The seed lot is destroyed (the pathogen is quarantined in Mexico).
- At ICARDA: Not applicable
References and further reading
Bruehl GW. 1957. Cephalosporium stripe disease of wheat. Phytopathology 47: 641-649.
Murray TD. 2006. Seed transmission of Cephalosporium gramineum in winter wheat. Plant Disease 90: 803-806.
Wiese MV. 1972. Colonization of wheat seedlings by Cephalosporium gramineum in relation to symptom development. Phytopathology 62: 1013-1018.
Wiese MV. 1987. Compendium of wheat diseases. 2nd edn. St Paul, MN: APS Press. pp. 26-28.
Scientific name
Magnaporthe oryzae Triticum patotype (Hebert) Barr (anamorph Pyricularia grisea (Cooke) Sacc.)
Importance
High phytosanitary importance. The disease is seed-transmitted, resistance is very limited, and spraying of fungicide to control wheat blast gives inconsistent results.
|
Wheat blast caused by M. grisea |
Significance
High economic importance. The disease has become a limiting factor in wheat production in Brazil (Urashima et al. 1993). It has caused a serious decline in wheat production in recent years in the lowlands of Bolivia. Losses of 10% have been reported, but 90-100% of spikes can be affected, resulting in near-complete losses, if favorable climatic conditions and critical growth stages for infection coincide.
Symptoms
Most damage results from infection of the rachis, causing partial or total sterility of spikes. Frequently, all the spikelets above the infection point turn white. However, all foliar parts may be infected.
Lesions on leaves, culms and glumes resemble those of rice blast, and are elliptical to elongated with white to light brown centers and dark gray to reddish-brown borders. Sporulation occurs on the underside of leaves.
Disease on seedlings can be severe, while the incidence of foliar lesions is generally lower the older the crop at infection. Seeds infected during early stages of development are severely shriveled and usually killed. However, those infected later may appear healthy and provide a primary source of disease inoculum.
Hosts
Wheat and tropical grasses.
According to Urashima et al. (1993) isolates from wheat did not infect rice, nor grasses such as Digitaria spp. or Brachiaria spp. The same isolates did produce symptoms on barley, maize, oat, rye, sorghum, and grasses such as Dactylis glomerata, Colium spp., and Festuca spp. Conversely, some isolates from rice were capable of infecting wheat.
Geographic distribution
Parts of Brazil, Bolivia and Paraguay. The disease is prevalent in warmer wheat-growing areas. Found on barley in Uruguay (S. German, personal communication).
Biology and transmission
Wheat blast is seed-transmitted. The transmission rate depends on the time of infection in relation to heading. Early infection leads to low seed-transmission rates. Transmission is greater when seed infection occurs later in the grain-filling period (Cunfer et al. 1993).
The wheat pathogen M. oryzae Triticum patotype found in Brazil does not originate from rice (Prabhu et al. 1992; Urashima 2005; Valent and Chumley 1994) and is different from the rice pathogen (Couch and Kohn 2002).
Detection/indexing methods used
- At CIMMYT: Freezing blotter test, Agar plate test (Warham et al. 1996).
- At ICARDA: Not applicable
Treatment/control
- Seed treatments effective against M. oryzae in rice are thought to have similar activity against M. oryzae Triticum patotype in wheat seed. Thiram + benomyl has been successfully used in Japan on rice.
- Iprodione + thiram has been reported as a very effective seed treatment in wheat (Igarashi 1990).
Procedures followed at the centers in case of positive test
- At CIMMYT: The pathogen is not present on wheat in Mexico, but it is not officially quarantined. If intercepted on seed introductions the seed is destroyed as per internal policy.
- At ICARDA: Not applicable
References and further reading
Cunfer BM, Yorinori T, Igarashi S. 1993. Wheat blast. In S.B. Mathur and B.M. Cunfer (eds.), Seed-borne Diseases and Seed Health Testing of Wheat. Copenhagen: Danish Government Institute of Seed Pathology for Developing Countries. pp. 125-128.
de Andrade Cardoso CA, Melo Reis E, Novaes Moreira E. 2008. Development of a warning system for wheat blast caused by Pyricularia grisea. Summa Phytopathol. 34: 216-221.
Dias Martins T, Lavorenti NA, Urashima AS. 2004. Comparação entre métodos de avaliação de transmissão de Pyricularia grisea através de sementes em triticale. Fitopatol. Bras. 29: 425-428.
Diekmann M, Putter CAJ. (eds.). 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14: Small Grain Temperate Cereals. Rome: Food and Agriculture Organization of the United Nations (FAO) and International Plant Genetic Resources Institute (IPGRI). pp. 51-52.
Goulart ACP, Paiva FA. 1991. Controle de Pyricularia oryzae e Helminthosporium sativum pelo tratamento de sementes de trigo com fungicidas. Pesquisa Agropecuária Brasileira 26: 1983-1988.
Goulart ACP, Paiva FA, Mesquita AN. 1989. Ocorrencia de brusone em trigo no Estado do Mato Grosso do Sul. Summa Phytopaphologica 15: 9.
Igarashi S. 1988. Analise da ocorrencia de brusone do trigo no Parana. In: Reuniao Nacional de Pesquisa de Trigo 15, Passo Fundo. Resumos. Passo Fundo: Embrapa-CNPT. 19 pp.
Igarashi S. 1990. Update on wheat blast (Pyricularia oryzae). In D.A. Saunders (ed.), Wheat for the Nontraditional Warm Areas: A Proceedings of the International Conference, July 29–August 3, 1990, Foz do Iguacu, Brazil. Mexico, D.F.: CIMMYT. pp. 480-483.
Igarashi S, Utiamada CM, Igarashi LC, Kazuma AH, Lopes RS. 1986. Pyricularia in wheat. 1. Occurrence of Pyricularia sp. in Paraná State. Fitopatol. Bras. 11: 351-352.
Igarashi S, Utiamada CM, Igarashi LC, Kazuma AH, Lopes RS. 1986. Pyricularia sp em trigo. Ocorrência de Pyricularia sp. no Estado do Paraná. In: Reunião Nacional de Pesquisa de Trigo 14, Londrina. Resumos. Londrina: IAPAR. p.57.
Menten JOM, Morais MHD. 1987. Pyricularia sp. in wheat seeds. Detection method, localization and transmission of the pathogen [in Portuguese]. In Resumos do V Congresso Brasileiro de Sementes. Gramado, Rio Grande de Sul, Brazil. p. 197.
Murakami J, Tosa Y, Kataoka T, Tomita R, Kawasaki J, Chuma I, Sesumi Y, Kusaba M, Nakayashiki H, Mayama S. 2000. Analysis of host species specificity of Magnaporthe grisea toward wheat using a genetic cross between isolates from wheat and foxtail millet. Phytopathology 90: 1060-1067.
Piccinini EC, Fernandes JMC. 1989. Ocorrencia da brusone (Pyricularia orizae) em lavouras comerciais de trigo (Triticum aestivum) no Estado do Rio Grande do Sul. Fitopatol. Bras. 14: 126.
Prabhu AS, Fillipi MC, Castro N. 1992. Pathogenic variation among isolates of Pyricularia oryzae affecting rice, wheat, and grasses in Brazil. Tropical Pest Management 38: 367-371.
Prestes AM, Arendt PF, Fernandes JMC, Scheeren PL. 2007. Resistance to Magnaporthe grisea among Brazilian wheat genotypes. In: Buck HT, Nisi JE, Salomón N. (eds.), Wheat Production in Stressed Environments : Proceedings of the 7th International Wheat Conference, 27 November - 2 December 2005, Mar Del Plata, Argentina. Developments in Plant Breeding 11. Dordrecht: Springer. pp. 119-123.
Reis EM, Blum MC, Forcelini CA. 1995. Sobrevivência de Pyricularia oryzae, associada a sementes de trigo. Summa Phytopathologica 21: 43-44.
Tosa Y, Hirata K, Tamba S, Nakagawa S, Chuma I, Isobe C, Osue J, Urashima AS, Don LD, Kusaba M, Nakayashiki H, Tanaka A, Tani T, Mori N, Mayama S. 2004. Genetic constitution and pathogenicity of Lolium isolates of Magnaporthe oryzae in comparison with host species-specific pathotypes of the blast fungus. Phytopathology 94: 454-462.
Urashima AS, Galbieri R, Stabili A. 2005. DNA finger printing and sexual characterization revealed two distinct populations of Magnaporthe grisea in wheat blast from Brazil. Czech J. Genet. Plant Breed. 41: 238-245.
Urashima AS, Igarashi S, Kato H. 1993. Host range, mating type, and fertility of Pyricularia grisea from wheat in Brazil. Plant Dis. 77: 1211-1216.
Valent B, Chumley FG. 1994. Avirulence genes and mechanisms of genetic instability in the rice blast fungus. In: Zeigler RS, Leong SA, Teng PS. (eds.), Rice Blast Disease. Wallingford, UK: CAB International. pp.111-134.
Zhan SW, Mayama S, Tosa Y. 2008. Identification of two genes for resistance to Triticum isolates of Magnaporthe oryzae in wheat: Genome 51: 216-221.
Pink snow mold, Michrodochium seedling blight, Fusarium leaf blotch, scab, ear blight
|
Fusarium leaf blotch (also known as Snow Mold). |
Scientific name
Monographella nivalis var. nivalis (Schaffnit) E. Müll., (anamorph. Microdochium nivale (Fr.) Samuels & I.C.) Hallett) and Microdochium majus Glynn & S.G. Edwards (2005)
Other scientific names
Fusarium nivale (Fr.) Ces. ex Berl. & Vogl. (teleomorph Calonectria nivalis (Schaffnit)).
The nomenclature has changed several times.
Importance
High. Present in Mexico.
Significance
High levels of seed infection and untreated seed sown into poor seedbeds are disease risk factors and may cause seedling blight and very poor crop establishment.
Also prevails with wet weather conditions at flowering.
These fungi are among the more than 18 fungi causing or associated with ear blight (also known as scab, Fusarium head blight).
Symptoms
The most common symptom of a serious attack is poor establishment.
Microdochium seedling blight occurs on wheat seeds and is observed in wet climates (e.g. the UK). N.B. Symptoms for M. nivalis and M. majus are the same.
In Mexico M. nivalis is observed in cool wet environments above 2600 masl and causes a leaf blotch, with oval-shaped, dull grayish-green lesions. These enlarge quickly into large, grayish-brown “eyespot” blotches with bleached or light gray centers. White to salmon-colored sporodochia develop rapidly on the lesions.
The fungi can also cause foot rot and pink snow mold in winter cereals.
Hosts
Wheat, triticale, maize, barley, rye, and numerous grasses.
Generally more severe on durum wheat and triticale than bread wheat or rye.
Geographic distribution
Worldwide.
Biology and transmission
The pathogens can be seedborne. Spores on seed, in soil, and on infected crop debris may act as inoculum. Spores produced on surface crop debris are transmitted to leaves by wind or rain splash.
The macronidia of M. nivale are 2.8-4 x 16-25 μm in size, 1 to 3 septate, without an evident heel; colonies are white to peach-colored. Microconidia and chlamydospores are absent.
M. majus is very common in cereals and has 3-7 septate macroconidia.
Seedling infection is favored by cool, dry soil and increasing depth of seeding. Disease severity is directly correlated with rainfall and duration of cool temperatures.
Detection/indexing methods used
- At CIMMYT: Freezing blotter test..
- At ICARDA: Freezing blotter test
Treatment/control
- Seed treatment with appropriate fungicide
Procedures followed at the centers in case of positive test
- At CIMMYT: Seed treatment with appropriate fungicide.
- At ICARDA: Seed treatment
References and further reading
Browne RA. 2007. Components of resistance to Fusarium head blight (FHB) in wheat detected in a seed-germination assay with Microdochium majus and the relationship to FHB disease development and mycotoxin accumulation from Fusarium graminearum infection. Plant Pathology 56: 65-72.
CIMMYT. Wheat Doctor information sheet: Fusarium leaf blotch. [online] Available from URL: http://wheatdoctor.cimmyt.org/. Date accessed 07 April 2010
CIMMYT. Wheat Doctor information sheet: Scab (head blight). [online] Available from URL: http://wheatdoctor.cimmyt.org/. Extended information sheet: Fusarium head blight. [online] Available from URL: http://wheatdoctor.cimmyt.org/. Date accessed 07 April 2010
Diekmann M, Putter CAJ. (eds.). 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14: Small Grain Temperate Cereals. Rome: Food and Agriculture Organization of the United Nations (FAO) and International Plant Genetic Resources Institute (IPGRI). pp. 31-33.
McBeath JH, Smith JD, Tronsmo AM. 1993. Pink snow mold, leaf blotch and ear blight. In S.B. Mathur and B.M. Cunfer (eds.), Seed-borne Diseases and Seed Health Testing of Wheat. Copenhagen: Danish Government Institute of Seed Pathology for Developing Countries. pp. 95-103.
Prescott JM, Burnett PA, Saari EE, Ransom J, Bowman J, de Milliano W, Singh RP, Bekele G. 1986. Wheat Diseases and Pests: A Guide for Field Identification. Mexico, D.F.: CIMMYT. pp. 30-31, 32-33, and 48-49. [online] Available from URL: http://www.cimmyt.org/. Date accessed 07 April 2010
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 12.
Wiese MV. 1987. Compendium of Wheat Diseases. 2nd edn. St. Paul, MN: APS Press. pp. 32-33.
Septoria glume blotch, Stagonospora nodorum blotch, Septoria nodorum blotch, glume blotch
|
P. nodorum lesions tend to be lens shaped
Septoria: Heavy infection can kill leaves, spikes, |
Scientific name
Phaeosphaeria nodorum (E. Müller) Hedjaroude (teleomorph): (anamorph Stagonospora nodorum (Berk.) Castellani and E.G. Germano)
Other scientific names
Leptosphaeria nodorum, Septoria nodorum
Importance
Moderate phytosanitary importance. Present in Mexico.
Significance
High economic importance.
Phaeosphaeria nodorum often occurs together with M. graminicola (anamorph Septoria tritici).
In recent decades Septoria tritici botch is more prevalent in many growing areas where Septoria glume blotch used to prevail.
Septoria glume blotch may cause up to 30% loss of yield in wheat, and worldwide annual yield losses to the disease of 2% are reported (Wiese 1987). Losses are dependent on the susceptibility of the wheat cultivar, weather conditions (the disease is favored by wet and windy conditions with moderate temperatures), and availability of inoculum on diseased stubble, on volunteer wheat or from the use of diseased seeds.
Symptoms
Phaeosphaeria nodorum infects the leaf and stem and causes discoloration of the head in the symptom known as glume blotch.
Water-soaked lesions can be observed on all aerial parts of the plant, most typically on the floral braces and nodal tissues of the culm.
On leaves, early symptoms include yellowing at the site of infection and tip burn of the leaf. The chlorotic front expands to form oval or lens-shaped lesions on the leaf, with the majority of hyphae running parallel to the vasculature of the leaf.
P. nodorum lesions are initially red-brown surrounded by a yellow halo, measuring 3-6mm by 6-19 mm, turning brown or gray-brown and necrotic with age.
Pycnidia (spore-producing structures) can form throughout the lesion, typically in necrotic areas. Pycnidia of P. nodorum are light to dark brown and circular, and three times larger than pycnidia of M. graminicola.
Hosts
S. nodorum has been isolated from hosts in 17 genera. Besides wheat, the pathogen occurs on barley, rye and other grasses, and on triticale. Agropyron and Bromus spp. are among the many alternative hosts.
Geographic distribution
Cosmopolitan. Septoria glume blotch can be found in all the world’s wheat-growing areas.
Biology and transmission
The disease is seed-transmitted. Windborne ascospores and seedborne pycnidiospores and mycelium are the main sources of primary inoculum. The seedborne mycelium can cause seedling infection. The pycnidiospores or ascospores germinate on wet leaf surfaces and enter the leaf tissue through stomata or directly through the epidermal tissue. P. nodorum infections occur over a wide temperature range, ideally between 20 to 27°C with wet and windy weather conditions. The fungus is able to infect all above-ground plant parts and overwinters in the field on infected stubble, where the sexual stage occurs.
The teleomorph stage is reported from Australia, Chile, South Africa, Ukraine and the USA.
Successive rounds of asexual reproduction and dispersal can distribute P. nodorum throughout the plant canopy.
Up to 16 hrs of wetness is required for infections to occur and within 10-20 days secondary spores are produced in lesions. Infection is highly dependent on the plant’s stage of development. It starts before head emergence and the disease becomes more severe later in the season. Further spread can lead to glume blotch as a result of direct attack of glumes by the fungus.
Pseudothecia and pycnidia on wheat stubble are also infectious. The presence of wheat stubble, volunteer wheat, susceptible grasses, and diseased seed allows the survival of the fungus from year to year. These become sources of the inoculum for infection of the new wheat crop.
The host-specific toxin ToxA produced by both the wheat pathogens P. tritici-repentis and P. nodorum interacts with the product of the dominant plant gene Tsn1 to induce necrosis around infection points. The ToxA gene is thought to have been acquired by P. tritici-repentis from P. nodorum through a recent horizontal gene transfer event.
Detection/indexing methods
- At CIMMYT: Freezing blotter test.
- At ICARDA: Freezing Blotter Test
Treatment/control
- Fungicide seed treatment: prochloraz, triazoles, or carbendazim.
- Application of fungicides such as difenoconazole or triadimenol at a suitable time will effectively control most outbreaks.
Procedures followed at the centers in case of positive test
- At CIMMYT: Seed treatment with carboxin and captan.
- At ICARDA: Seed treatment
ISTA protocol
ISTA. International Rules for Seed Testing: 7-014: Detection of Septoria nodorum on Triticum aestivum (Wheat). [online] Available from URL: https://www.seedtest.org/upload/cms/user/7-014.pdf. Date accessed 06 April 2010
References and further reading
Aguilar V, Stamp P, Winzeler M, Winzeler H, Schachermayr G, Keller B, Zanetti S, Messmer MM. 2005. Inheritance of field resistance to Stagonospora nodorum leaf and glume blotch and correlations with other morphological traits in hexaploid wheat (Triticum aestivum L.). Theor. Appl. Genet. 111: 325-336.
CIMMYT. Wheat Doctor information sheet: Septoria. [online] Available from URL: http://wheatdoctor.cimmyt.org/. Extended information sheet: Septoria glume blotch. [online] Available from URL: http://wheatdoctor.cimmyt.org. Date accessed 06 April 2010
Cunfer BM. 1993. Leaf and glume blotch. In S.B. Mathur and B.M. Cunfer (eds.), Seed-borne Diseases and Seed Health Testing of Wheat. Copenhagen: Danish Government Institute of Seed Pathology for Developing Countries. pp. 73-81.
Diekmann M, Putter CAJ. (eds.). 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14: Small Grain Temperate Cereals. Rome: Food and Agriculture Organization of the United Nations (FAO) and International Plant Genetic Resources Institute (IPGRI). pp. 44-45.
Duzcek LJ, Sutherland KA, Reed SL, Bailey KL, Lafond GP. 1999. Survival of leaf spot pathogens on crop residues of wheat and barley in Saskatchewan. Can. J. Plant Pathol. 21: 165-173.
Eyal Z. 1999. Septoria and Stagonospora diseases of cereals: a comparative perspective. In: J.A. Lucas, P. Bowyer, and H.M. Anderson (eds.), Septoria on Cereals: A Study of Pathosystems. Wallingford, UK: CABI. pp. 1-25.
Gilchrist L, Dubin HJ. 2002. Septoria diseases of wheat. In: Curtis BC, Rajaram S, Gómez Macpherson H. (eds.), Bread Wheat: Improvement and Production. FAO Plant Production and Protection Series No. 30. Rome: Food and Agriculture Organization of the United Nations (FAO). [online] Available from URL: http://www.fao.org/. Date accessed 06 April 2010
HGCA Cereal Disease Encyclopedia: Leaf and glume blotch. [online] Available from URL: http://www.hgca.com/. Date accessed 06 April 2010
Kema GHJ, van Ginkel M, Harrabi M. (eds.). 2003. Global Insights into the Septoria and Stagonospora Diseases of Cereals: Proceedings of the Sixth International Symposium on Septoria and Stagonospora Diseases of Cereals. Tunis, Tunisia.
Loughman R, Lagudah ES, Trottet M, Wilson RE, Mathews A. 2001. Septoria nodorum blotch resistance in Aegilops tauschii and its expression in synthetic amphiploids. Aust. J. Agr. Res. 52: 1393-1402.
Mercado-Vergnes D, Zhanarbekova A, Renard ME, Duveiller E, Maraite H. 2006. Mating types of Phaeosphaeria nodorum (anamorph Stagonospora nodorum) from Central Asia. Journal of Phytopathology 154: 317-319.
Prescott JM, Burnett PA, Saari EE, Ransom J, Bowman J, de Milliano W, Singh RP, Bekele G. 1986. Wheat Diseases and Pests: A Guide for Field Identification. Mexico, D.F.: CIMMYT. pp 20-23. [online] Available from URL: http://www.cimmyt.org/. Date accessed 06 April 2010
Solomon PS, Lowe RG, Kar-Chun T, Waters ODC, Oliver RP. 2006. Stagonospora nodorum: cause of Stagonospora nodorum blotch of wheat. Molecular Plant Pathology 7: 147-156.
Sommerhalder RJ, McDonald BA, Zhan J. 2006. The frequencies and spatial distribution of mating types in Stagonospora nodorum are consistent with recurring sexual reproduction. Phytopathology 96: 234-239.
Stukenbrock EH, McDonald BA. 2007. Geographical variation and positive diversifying selection in the host-specific toxin SnToxA. Molecular Plant Pathology 8: 321-332.
Stukenbrock EH, Banke S, McDonald BA. 2006. Global migration patterns in the fungal wheat pathogen Phaeosphaeria nodorum. Molecular Ecology 15: 2895–2904.
Wallwork H. 2000. Cereal Leaf and Stem Diseases. Adelaide: Grains Research and Development Corporation. pp.16-17.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. pp. 50-51.
Wiese MV. 1987. Compendium of wheat diseases. 2nd edn. St Paul, MN: APS Press. pp. 43-45.
Tan spot, yellow leaf spot
|
Tan spot (also known as Yellow leaf spot or |
Scientific name
Pyrenophora tritici-repentis (Died.) Drechsler, (anamorph Drechslera tritici-repentis (Died.) Shoemaker)
Other scientific names
Helminthosporium tritici-repentis, Pyrenophora trichostoma
Importance
Moderate phytosanitary importance. Present in Mexico. Seed transmission is often overlooked.
Significance
High economic importance due to the expansion of zero tillage.
Losses are often underestimated because the pathogen is overlooked. Pyrenophora tritici-repentis is a stubble-borne pathogen significant in areas where wheat residues are retained, and is thus important under conservation agriculture. Yield losses may reach approximately 50% in Australia (Rees and Platz 1990).
Red smudge is a discoloration caused by P. tritici-repentis under prolonged wet periods during kernel development, and can result in market discounts.
Symptoms
Tan-brown flecks, expanding into lens-shaped lesions up to 12 mm long. Often surrounded by a yellow border and with a dark brown spot in the centre. Lesions may coalesce, causing large necrotic areas in older leaves. In severe cases premature leaf death causes a high degree of kernel shriveling.
On susceptible wheat cultivars tan necrosis and/or a more or less extensive chlorosis may be observed (Lamari and Bernier 1989). Currently, a race-designation system based on necrosis and/or chlorosis symptoms induced on a wheat differential set distinguishes at least eight races. These symptoms are induced by host selective toxins (HSTs): necrosis is induced by Ptr ToxA (synonyms: Ptr necrosis toxin, Ptr toxin, ToxA) and chlorosis is induced on certain wheat differential genotypes by the production of either Ptr ToxB (synonym: Ptr chlorosis toxin) or Ptr ToxC (Lamari et al. 1995; Ciufetti et al. 1998).
Red smudge, a reddish discoloration of the kernels, results from prolonged wet periods and high humidity during kernel development. Red smudge-affected kernels are generally plump rather than shriveled.
Hosts
Natural: wheat, rye, Bromus spp., Agropyron spp. (Wiese 1987); barley.
Experimental: many grass species (Krupinsky 1992).
Geographic distribution
Worldwide.
Biology and transmission
Pyrenophora tritici-repentis has been reported as a homothallic (self-fertile) loculoascomycete, meaning that it does not require a partner to mate. In nature the pathogen completes a single sexual cycle during the growing season after overwintering on the stubble left in the field, whereas several cycles of asexual reproduction can occur during the same period. Conidia develop on lesions and serve as secondary inoculum; their production and release are favored by rain or dew. The pathogen overwinters on stubble left in the field, surviving saprophytically as mycelia and pseudothecia in or on infested host debris under wet conditions. Ascospores, conidia, and mycelia serve as primary inoculum, whereas seedborne inoculum does occur but appears less significant.
Detection/indexing methods used
- At CIMMYT: Freezing blotter test.
- At ICARDA: Freezing blotter test
Treatment/control
- Effective fungicides are available for seed treatment, and can reduce the risk of seedling infections that might arise from planting seed with red smudge. Fungicides are also available for both early season control of tan spot and for later season control. However, the list of approved active ingredients may vary according to countries. Locally systemic fungicides, such as triazoles and strobilurins, have very good to excellent activity against the leaf spot diseases of wheat. Products with mixtures of these two classes of chemical are also are available.
Procedures followed at the centers in case of positive test
- At CIMMYT: Seed treatment with appropriate fungicide.
- At ICARDA: Seed treatment
References and further reading
Andrie RM, Pandelova I, Ciuffetti LM. 2007. A combination of phenotypic and genotypic characterization strengthens Pyrenophora tritici-repentis race identification. Phytopathology 97: 694-701.
CIMMYT. Wheat Doctor information sheet: Tan spot. [online] Available from URL: http://wheatdoctor.cimmyt.org/. Date accessed 06 April 2010
Extended information sheet: Tan spot. [online] Available from URL: http://wheatdoctor.cimmyt.org/. Date accessed 06 April 2010
Ciuffetti LM, Francl LJ, Ballance GM, Bochus WW, Lamari L, Meinhardt SW, Rasmussen JB. 1998. Standardization of toxin nomenclature in the Pyrenophora tritici-repentis/wheat interaction. Can. J. Plant Pathol. 20: 421-424.
Cuiffetti LM, Tuori RP. 1999. Advances in the characterization of the Pyrenophora tritici-repentis-wheat interaction. Phytopathology 89: 444-449.
De Wolf ED, Effertz RJ, Ali S, Francl LJ. 1998. Vistas of tan spot research. Can. J. Plant Pathol. 20: 349-370.
Diekmann M, Putter CAJ. (eds.). 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14: Small Grain Temperate Cereals. Rome: Food and Agriculture Organization of the United Nations (FAO) and International Plant Genetic Resources Institute (IPGRI). pp. 40-41.
Duveiller E, Dubin HJ, Reeves J, McNab A. (eds.). 1998. Helminthosporium Blights of Wheat: Spot Blotch and Tan Spot. Proceedings of the International Workshop on Helminthosporium Diseases of Wheat: Spot Blotch and Tan Spot, El Batán, Feb 9-14, 1997. Mexico D.F.: CIMMYT. 376pp.
Fernandez MR, DePauw RM, Clarke JM, Fox SL. 1998. Discoloration of wheat kernels by Pyrenophora tritici-repentis. Can. J. Plant Pathol. 20: 380-383.
Krupinsky JM. 1992. Grass hosts of Pyrenophora tritici-repentis. Plant Disease 76: 92-95.
Lamari L, Bernier CC. 1989. Virulence of isolates of Pyrenophora tritici-repentis on 11 wheat cultivars and cytology of the differential host reactions. Can. J. Plant Pathol. 11: 284-290.
Lamari L, Sayoud R, Boulif M, Bernier CC. 1995. Identification of a new race in Pyrenophora tritici-repentis: implications for the current pathotype classification system. Can. J. Plant Pathol. 17: 312-318.
Lamari L, Strelkov SE, Yahyauoi A, Amedov M, Saidov M, Djunusova M. 2005. Virulence of Pyrenophora tritici-repentis in the countries of the silk road. Can. Jour. Plant Pathol. 27: 383-388.
Moreno MV, Stenglein SA, Balatti PA, Perelló AE. 2008. Pathogenic and molecular variability among isolates of Pyrenophora tritici-repentis, causal agent of tan spot of wheat in Argentina. Eur. J. Plant Pathol. 122: 239-252.
Prescott JM, Burnett PA, Saari EE, Ransom J, Bowman J, de Milliano W, Singh RP, Bekele G. 1986. Wheat Diseases and Pests: A Guide for Field Identification. Mexico, D.F.: CIMMYT. pp. 26-27. [online] Available from URL: http://www.cimmyt.org/. Date accessed 06 April 2010
Rees RG, Platz GJ. 1990. Sources of resistance to Pyrenophora tritici-repentis in bread wheats. Euphytica 45: 59-69.
Schilder AMC, Bergstrom GC. 1992. Seed transmission of Pyrenophora tritici-repentis, causal fungus of tan spot of wheat. Eur. J. Plant Pathol. 101: 81-91.
Strelkov SE, Lamari L. 2003. Host-parasite interactions in tan spot (Pyrenophora tritici-repentis) of wheat. Can. J. Plant Pathol. 25: 339-349.
Wiese MV. 1987. Compendium of wheat diseases. 2nd edn. St Paul, MN: APS Press. p. 42.
Zhanarbekova AB, Mercado D, Maraite H, Duveiller E, Renard ME, Morgounov AI, Koishibayev M. 2007. Tan spot of wheat in Kazakhstan and resistance of winter wheats to four races of Pyrenophora tritici-repentis. Agromeridian 1(5): 24-28.
|
If downy mildew is formed, the heads may be
Here you see that on a diseased plant, the leaves |
Scientific name
Sclerophthora macrospora (Sacc.) Thirm., Shaw & Naras.
Other scientific names
Sclerospora macrospora Sacc., Phytophthora macrospora (Sacc.) Ito & Tanaka
Importance
Present in Mexico. Reported to be seedborne
Significance
The disease occurs occasionally in wet areas, with small, localized epidemics when conditions are favorable. The disease is not common and occurs with low frequency on individual plants. There have been no reports of widespread and destructive epidemics. However, it warrants careful monitoring of its presence and spread. It can survive for months in well-drained soils and in grasses as oospores. It can be controlled by proper water management, weed control and rotation.
Symptoms
In young plants, the disease causes yellowing, severe stunting, and profuse tillering. Many plant die at this stage. Tillers die prematurely or never head.
Older plants show short, erect, irregular or crooked, yellowish-green culms. The leaves are thick and twisted, erect, and usually in whorls. If formed, the heads may be branched, and some of the floral tissues grow into leaf-like structures. The diseased ears show ‘crazy top’ symptoms with varying abnormalities.
The diagnostic feature is the presence of numerous spherical yellowish-brown oospores in hypertrophied and hyperplastic diseased parts.
Hosts
The fungus has a broad host range, including small grain cereals, maize, sorghum, and most grasses.
Geographic distribution
Cosmopolitan. The disease may be found wherever soils become waterlogged or are poorly drained.
Biology and transmission
The pathogen is an obligate parasite. The primary source for new infections is oospores, which remain viable for years in soil, dead plant tissue and seeds. Germinating oospores produce sporangiophores, which germinate to release motile zoospores. Under free moisture conditions these can systemically infect plants at any state of development.
Detection/indexing methods used
- At CIMMYT: Seed transmission in wheat is reported only under controlled conditions; plants with downy mildew infection do not usually produce seed.
- At ICARDA: Not applicable
Treatment/control
- No additional specific seed treatments can be recommended.
- Use seed from disease-free crop.
Procedures followed at the centers in case of positive test
- At CIMMYT: Not applicable.
- At ICARDA: Not applicable.
References and further reading
Chahal SS, Singh PP. 1993. Downy mildew. In S.B. Mathur and B.M. Cunfer (eds.), Seed-borne Diseases and Seed Health Testing of Wheat. Copenhagen: Danish Government Institute of Seed Pathology for Developing Countries. pp. 69-71.
CIMMYT. Wheat Doctor information sheet: Downy mildew. [online] Available from URL: http://wheatdoctor.cimmyt.org/. Date accessed 06 April 2010
Diekmann M, Putter CAJ. (eds.). 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14: Small Grain Temperate Cereals. Rome: Food and Agriculture Organization of the United Nations (FAO) and International Plant Genetic Resources Institute (IPGRI). p. 30.
Prescott JM, Burnett PA, Saari EE, Ransom J, Bowman J, de Milliano W, Singh RP, Bekele G. 1986. Wheat Diseases and Pests: A Guide for Field Identification. Mexico, D.F.: CIMMYT. pp. 38-39. [online] Available from URL: http://www.cimmyt.org/ Date accessed 06 April 2010
Wiese MV. 1987. Compendium of Wheat Diseases. 2nd edn. St. Paul, MN: APS Press. pp. 35-37.
|
Common and dwarf bunt (also known as Stinking |
Scientific name
Tilletia controversa Kühn
Importance
High phytosanitary importance.
Significance
Considerable yield losses can occur when susceptible cultivars are grown or chemical seed treatments are not used. The disease is prevalent in areas with temperatures just above freezing for several months of the year, and snow cover. In West Asia and North Africa it occurs only at altitudes above 1100 m. Bunted spikes result only from high levels of seed infestation (more than 20,000 teliospores per seed).
Dwarf bunt is a serious disease, particularly of winter wheat at relatively high altitudes. It is very difficult to control because of resistant resting spores, which remain viable in the soil for a number of years, and because most seed-applied fungicides are not effective.
Symptoms
Affected plants are stunted to various extents. Tillering may be increased. Kernel symptoms resemble those of common bunt. The main symptoms are fungal structures called “bunt balls,” which resemble kernels but are completely filled with black teliospores. The bunt balls of dwarf bunt are more nearly spherical than those of common bunt. When bunt balls are crushed they give off a fetid or fishy odor. Infected spikes tend to be bluish green in color (or darker), and the glumes tend to spread apart slightly; the bunt balls often become visible after the soft dough stage.
Hosts
Wheat, rye, triticale (less commonly), barley, and a number of grasses (Aegilops spp., Agropyron spp., Alopecurus myosuroides, Arrhenatherum elatius, Bromus spp. Dactylis glomerata, Elymus spp., Festuca spp., Koeleria cristata, Lolium spp., Poa spp.).
Geographic distribution
Dwarf bunt can occur worldwide. It is limited to temperate climates in areas where winter cereals are grown with prolonged snow cover, including parts of Europe, the USA, Canada, the Near East.
Biology and transmission
The pathogen is both soilborne and externally seedborne, with the majority of dwarf bunt infections resulting from soil infestation. Teliospores can remain viable for 3 to 10 years in soil or on seeds.
Dwarf bunt spores require low temperatures (optimum 0-8°C) over a period of several weeks to germinate. This normally occurs under snow cover on unfrozen ground during the winter months.
Detection/indexing methods used
- At CIMMYT: Physical inspection of seed, Seed washing and filtration test, Fluorescence microscopy may help to distinguish the spores from those of T. tritici: the reticulated wall layer of T. controversa teliospores fluoresces yellow-orange.
- At ICARDA: Dry visual inspection, Centrifuge wash test
Treatment/control
- Seed can be treated with systemic fungicides such as Difenoconazole or NaOCl (0.13 M at maximum 55°C).
- Soilborne inoculum is generally difficult to control; spores of the fungus can survive for more than 10 years in the soil.
Procedures followed at the centers in case of positive test
- At CIMMYT: The seed lot is destroyed (the pathogen is quarantined in Mexico).
- At ICARDA: The seed lot is destroyed.
References and further reading
CIMMYT. Wheat Doctor information sheet: Common and dwarf bunt. [online] Available from URL: http://wheatdoctor.cimmyt.org.
Extended information sheet: Common and dwarf bunt. [online] Available from URL: http://wheatdoctor.cimmyt.org/ Date accessed 06 April 2010
Chastain TG. 1991. High-temperature sodium hypochlorite effects on viability of Tilletia controversa teliospores and wheat seed. Crop Science 31:1327-1330.
Diekmann M, Putter CAJ. (eds.). 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14: Small Grain Temperate Cereals. Rome: Food and Agriculture Organization of the United Nations (FAO) and International Plant Genetic Resources Institute (IPGRI). pp. 26-27.
Peterson GL, Whitaker TB, Stefanski RJ, Podleckis EV, Phillips JG, Wu JS, Martinez WH. 2009. A Risk Assessment Model for Importation of United States Milling Wheat Containing Tilletia contraversa Plant Disease. 93: 560-573
Prescott JM, Burnett PA, Saari EE, Ransom J, Bowman J, de Milliano W, Singh RP, Bekele G. 1986. Wheat Diseases and Pests: A Guide for Field Identification. Mexico, D.F.: CIMMYT. pp. 8-11. [online] Available from URL: http://www.cimmyt.org/. Date accessed 06 April 2010
Sitton JW, Line RF, Waldher JT, Goates BJ. 1993. Difenoconazole seed treatment for control of dwarf bunt of winter wheat. Plant Disease 77: 1148-1151.
Stockwell VO, Trione EJ. 1986. Distinguishing teliospores of Tilletia controversa from those of T. caries by fluorescence microscopy. Plant Disease 70: 924-926.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 60.
Wiese MV. 1987. Compendium of wheat diseases. 2nd edn. St Paul, MN: APS Press. pp. 20-21.
Xue AG, Tenuta A, Tian XL, Sparry E, Etienne M, Gaudet DA, Menzies JG, Graf RJ, Falk DE, Smid A, Pandeya RS. 2007. Evaluation of winter wheat genotypes and seed treatments for control of dwarf bunt in Ontario. Can. J. Plant Pathol. 29: 243-250.
Karnal bunt, partial bunt
|
Karnal Bunt: As shown here, infected seeds |
Scientific name
Tilletia indica Mitra
Other scientific names
Neovossia indica (Mitra) Mundkur
Importance
High phytosanitary importance; pathogen is quarantined in more then 80 countries. Present in parts of Afghanistan, India, Iraq, Pakistan, Mexico, and the USA.
Significance
Karnal bunt is a relatively minor disease regarded as being of moderate economic importance everywhere it occurs. Actual yield losses are minimal, but since the disease is on the quarantine lists of many countries it is important in world grain trade and its indirect economic costs are more significant due to the quarantine measures that must be applied.
Symptoms
Karnal bunt is not easily detected prior to harvest, since it is usual for only a few kernels per spike to be affected by the disease. Following harvest, diseased kernels can be easily detected by visual inspection: a mass of black teliospores replaces a portion of the endosperm, and the pericarp may be intact or ruptured. Diseased kernels give off a fetid or fishy odor when crushed.
Hosts
Karnal bunt can affect wheat, triticale, rye, and several other related grasses, but not barley. Durum wheat and triticale are less susceptible than bread wheat.
Geographic distribution
The disease is endemic in the Asian Subcontinent and now in Mexico. It was intercepted in the USA in 1996.
EPPO distribution map: http://www.eppo.org/
Biology and transmission
Karnal bunt is a seed- or soilborne, floral-infecting disease. The primary sources of inoculum are seed and teliospores present in soil. Teliospores germinate at or near the soil surface, producing sporidia, in response to temperature (8-20°C) and moisture (high relative humidity associated with light rain showers and cloudy weather). These sporidia, or the haploid secondary allantoid sporidia produced subsequently, are carried to the floral structures by wind currents and/or splashing rain droplets. The sporidia then germinate and penetrate the glumes, rachis, or the ovary itself. Plants are infected within 2-3 weeks of heading. The fungus enters the newly-formed kernel and develops in the intercellular space between the endosperm and seed coat. Spikelets are also locally infected at the embryo end by diploid mycelia produced by the fusion of two mating types. Depending on the weather and the stage of seed maturity, the pathogen may become partially systemic in the rachis and rachillae and produce additional infections. The degree of disease establishment and development depends on environmental conditions from spike emergence to grain filling. Infection is favored by rainfall, cloud cover, heavy dew formation and cool temperatures (15-20°C) around the time of flowering.
Teliospores can be carried over long distances by wind, and can pass through the digestive tracts of animals undamaged. However, the main mode of international spread is through infected or contaminated wheat seed. Typically only a few kernels within the spike are affected by the disease, and infections are not noticed until after harvest. Kernels themselves are usually partially infected; hence the disease is also known as partial bunt.
Detection/indexing methods used
At CIMMYT:
- Physical inspection of seed.
- Infected seed may be seen with the naked eye or with the aid of a low-power magnifying glass. Detection may be improved by augmenting the contrast between the dark sori and the lighter uninfected areas by soaking seeds for 24 hours in a solution of 0.1% NaOH. Common black point is often confused with Karnal bunt. Always confirm identification by the presence of teliospores.
- Seed washing and filtration test
- Teliospores are characteristically large, more or less spherical, and dark (immature ones are lighter in colour). The mean diameter, including a sheath, ranges between 33 and 40 μm. Conidia of Epicoccum spp. are often confused with teliospores of T. indica; however, they are reticulated and only about 25 μm in size.
At ICARDA: Dry visual inspection, Karnal bunt test
Treatment/control
- Chemical seed treatments that reduce teliospore germination of T. indica appear to be limited in how long they remain effective, and, with the possible exception of the mercurial compounds, none is capable of killing teliospores when applied to infected seed.
- Pentachloronitrobenzene (PCNB) or chlorothalonil have given the best results as seed treatments, although all fungicides tested thus far are fungistatic to some degree. Further, teliospores protected by the seed pericarp and/or spore masses inside intact sori may be protected from the chemical. However, teliospores on seed surfaces are eliminated by disinfecting seeds in a 1% solution of sodium hypochlorite (+ Tween 20) with agitation for 3 min. Chlorothalonil and carboxin + thiram have been used as seed treatments in the USA and Mexico.
- Foliar spraying with propiconazole, triadimefon and carbendazim can control the incidence of T. indica on wheat.
Procedures followed at the centers in case of positive test
- At CIMMYT: The seed lot is destroyed (the pathogen is quarantined in Mexico).
- At ICARDA: The seed lot is destroyed.
Other protocols and best practices
EPPO Phytosanitary Procedure PM 3/37(1): Tilletia indica. Inspection and test methods for wheat seeds. [online] Available rfrom URL: http://archives.eppo.org/EPPOStandards Date accessed 06 April 2010
EPPO Diagnostic Protocols for Regulated Pests PM 7/29: Tilletia indica. EPPO Bulletin 34: 155-157. [online] Available from URL: http://www.eppo.org/QUARANTINE Date accessed 06 April 2010
NAPPO Standard for Phytosanitary Measures RSPM #13: Guidelines to establish, maintain and verify Karnal bunt pest free areas in North America. [online] Available from URL: http://www.nappo.org/ Date accessed 06 April 2010
NAPPO Standard for Phytosanitary Measures RSPM #21: A harmonized procedure for morphologically distinguishing teliospores of Karnal bunt, ryegrass bunt and rice bunt. [online] Available from URL: http://www.nappo.org/ Date accessed 06 April 2010
References and further reading
Agarwal VK, Singh DV, Mathur SB. 1993. Karnal bunt. In: Mathur SB, Cunfer BM. (eds.), Seed-borne Diseases and Seed Health Testing of Wheat. Copenhagen: Danish Government Institute of Seed Pathology for Developing Countries. pp. 31-43.
Bonde MR, Nester SE, Olsen MW, Berner DK. 2004. Survival of teliospores of Tilletia indica in Arizona field soils. Plant Disease 88: 804-810.
Brennan JP, Warham EJ, Byerlee D, Hernandez-Estrada J. 1992. Evaluating the economic impact of quality-reducing, seed-borne diseases: Lessons from Karnal bunt of wheat. Agricultural Economics 6: 345-352.
Carris LM, Castlebury LA, Goates BJ. 2006. Nonsystemic bunt fungi—Tilletia indica and T. horrida: A review of history, systematics, and biology. Annu. Rev. Phytopathol. 44: 113-33.
CIMMYT. Wheat Doctor information sheet: Karnal bunt. [online] Available from URL: http://wheatdoctor.cimmyt.org/ Date accessed 06 April 2010
Diekmann M, Putter CAJ. (eds.). 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14: Small Grain Temperate Cereals. Rome: Food and Agriculture Organization of the United Nations (FAO) and International Plant Genetic Resources Institute (IPGRI). pp 28-29.
EPPO. Data Sheets on Quarantine Pests: Tilletia indica. Online at: http://www.eppo.org/QUARANTINE/fungi/Tilletia_indica/NEOVIN_ds.pdf.
Fuentes-Davila G. 1996. Karnal bunt of wheat. In R.D. Wilcoxson and E.E. Saari (eds.), Bunt and Smut Diseases of Wheat: Concepts and Methods of Disease Management. Mexico, D.F.: CIMMYT. pp. 26-32.
Fuentes-Davila G. 1996. Karnal bunt in Mexico. Transcripts of the American Phytopathological Society Karnal Bunt Symposium. June 24–August 16, 1996. [online] Available from URL: http://www.apsnet.org/ Date accessed 06 April 2010
Fuentes-Davila G. 1997. Karnal bunt of wheat. CIMMYT webpage.
Goates BJ, Jackson EW. 2006. Susceptibility of wheat to Tilletia indica during stages of wheat development. Phytopathology 96: 962-966.
Jones DR. 2009. Towards a more reasoned assessment of the threat to wheat crops from Tilletia indica, the cause of Karnal bunt disease. Eur. J. Plant Pathol. 123: 247-259.
Nagarajan S, Aujla SS, Nanda GS, Sharma I, Goel LB, Kumar J, Singh DV. 1997. Karnal bunt (Tilletia indica) of wheat—a review. Review of Plant Pathology 76: 1207-1214.
Prescott JM, Burnett PA, Saari EE, Ransom J, Bowman J, de Milliano W, Singh RP, Bekele G. 1986. Wheat Diseases and Pests: A Guide for Field Identification. Mexico, D.F.: CIMMYT. pp. 12-13. [online] Available from URL: http://www.cimmyt.org/ Date accessed 06 April 2010
Sansford CE, Baker RHA, Brennan JP, Ewert F, Gioli B, Inman A, Kinsella A, Magnus HA, Miglietta FA, Murray GM, Porta-Puglia A, Porter JR, Rafoss T, Riccioni L, Thorne F. 2008. The new Pest Risk Analysis for Tilletia indica, the cause of Karnal bunt of wheat, continues to support the quarantine status of the pathogen in Europe. Plant Pathology 57: 603-611.
Stein JM, Maples HW, Rush CM. 2005. Epidemiology of Tilletia indica in regulated wheat fields in Texas. Plant Disease 89: 828-833.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 61.
Warham EJ. 1986. Karnal bunt disease of wheat: A literature review. Tropical Pest Management 32: 229-242.
Workneh F, Allen TW, Nash GH, Narasinhan B, Srinivasan R, Rush CM. 2008. Rainfall and temperature distinguish between Karnal bunt positive and negative years in wheat fields in Texas. Phytopathology 98: 95-100.
Ykema RE, Floyd JP, Palm ME, Petersen GL. 1996. First report of Karnal bunt of wheat in the United States. Plant Disease 80: 1207.
Zhang Z, Lange L, Mathur SB. 1984. Teliospore survival and plant quarantine significance of Tilletia indica (causal agent of Karnal bunt) particularly in relation to China. EPPO Bulletin 14: 119-128.
Common bunt, stinking smut.
|
Spores of Tilletia laevis under fluorescence microscopy.(photo: CIMMYT)
Common bunt (also known as Stinking Smut)
Spores of Tilletia tritici (syn. T. caries) under |
Scientific names
Tilletia tritici (Bjerk.) G. Wint. and Tilletia laevis Kühn
Other scientific names
Tilletia caries (DC.) Tul. & C. Tul (for T. tritici) and Tilletia foetida (Wallr.) Liro (for T. laevis)
Importance
Moderate phytosanitary importance.
Significance
High economic importance. Common bunt reduces yield and grain quality. It has a still greater effect on the value of wheat, as contaminated grain has an objectionable odor and cannot be used for human consumption. Common bunt is potentially important in most wheat-growing areas of the world. The most common source of infection is via sowing of contaminated seed from the previous crop.
The disease is ranked second after rusts in worldwide importance (Wiese 1987). It is of major importance where seed treatment is not practiced. In Syria, 50% of fields surveyed between 1984 and 1988 showed common bunt infection, with many of the fields exhibiting a high incidence (up to 60%) of infected plants (Mamluk and Zahour 1993).
Symptoms
Common bunt in wheat is caused by Tilletia tritici and T. laevis. The teliospores of T. tritici have a reticulate pattern, whereas the teliospores of T. laevis are smooth. T. tritici is the more common of the two fungi. Both fungi may occur at the same time in the same infected plant.
Obvious symptoms are not apparent until stem elongation starts. The main symptom of common bunt is the production of fungal structures called “bunt balls”, which replace healthy kernels. Bunt balls resemble kernels but are dark in color, containing masses of black powdery teliospores.
Infected spikes are bluish-green in colour and tend to maintain their green color longer than healthy heads. During harvest or upon crushing, the bunt balls rupture and release spores, resulting in the contamination of the wheat grain. The spores produce a pungent, fishy odor.
Infected kernels are sometimes confused with kernels infected by Anguina tritici (ear cockle), but the latter are smaller and do not break easily.
Hosts
Wheat, triticale, rye, barley and wild grasses.
Geographic distribution
Cosmopolitan.
Biology and transmission
Common bunt is primarily seedborne; seed may be naturally contaminated with as many as 230,000 spores/kernel. The disease may also be soilborne, with teliospores able to survive for at least two years in dry soil.
Teliospores both in the soil and on contaminated seed germinate at the same time as wheat seed. Teliospore germination depends largely on soil temperature and moisture, though spores germinate over a wide temperature range of 5-20°C.
The fungus invades the coleoptiles of the developing seedlings prior to emergence. The coleoptile is susceptible only as long as it is not split open. The fungus then invades deeper and establishes itself in the tissues that eventually develop into the head. In susceptible cultivars the fungus proliferates in the developing kernels as the plant grows and displaces the tissues within the kernels. During harvest teliospores are released from mature bunt balls. These teliospores are dispersed by wind and contaminate seeds and soils, completing the disease cycle and enabling the distribution of new strains of bunt fungi. Dispersal of common bunt is mainly by movement of grain and farm machinery contaminated with disease spores.
Smut and bunts are typical examples of monocyclic plant diseases. Losses are linearly dependant on infected tillers.
Detection/indexing method
- At CIMMYT: Physical inspection of seed, Seed washing and filtration test.
- At ICARDA: Visual inspection and centrifuge wash test
Treatment/control
- Chemical seed treatment, such as carboxin alone and in combination with thiram. However, soilborne inoculum may not be adequately controlled.
Procedures followed at the centers in case of positive test
- At CIMMYT: Seed treatment with carboxin, captan and chlorothalonil.
- At ICARDA: Water washing, drying and seed treatment
References and further reading
CIMMYT. Wheat Doctor information sheet: Common and dwarf bunt. [online] Available from URL: http://wheatdoctor.cimmyt.org/ Extended information sheet: Common and dwarf bunt. [online] Available from URL: http://wheatdoctor.cimmyt.org/ Date accessed 06 April 2010
Diekmann M, Putter CAJ. (eds.). 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14: Small Grain Temperate Cereals. Rome: Food and Agriculture Organization of the United Nations (FAO) and International Plant Genetic Resources Institute (IPGRI). pp 24-25.
Goates BJ. 1996. Common bunt and dwarf bunt. In: Wilcoxson RD, Saari EE. (eds.), Bunt and Smut Diseases of Wheat: Concepts and Methods of Disease Management. Mexico, D.F.: CIMMYT. pp. 12-25.
Line RF. 1993. Common bunt. In: Mathur Sb, Cunfer BM. (eds.), Seed-borne Diseases and Seed Health Testing of Wheat. Copenhagen: Danish Government Institute of Seed Pathology for Developing Countries. pp. 45-52.
Mamluk OF, Zahour A. 1993. Differential distribution and prevalence of Tilletia foetida (Wall.) Liro and T. caries (DC.) Tul. on bread wheat and durum wheat. Phytopathol. Medit. 32: 25-32.
Prescott JM, Burnett PA, Saari EE, Ransom J, Bowman J, de Milliano W, Singh RP, Bekele G. 1986. Wheat Diseases and Pests: A Guide for Field Identification. Mexico, D.F.: CIMMYT. pp 8-11. [online] Available from URL: http://www.cimmyt.org/ Date accessed 06 April 2010
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 59.
Wiese MV. 1987. Compendium of Wheat Diseases. 2nd edn. St. Paul, MN: APS Press. pp. 19-20.
|
Flag smut: Showing the epidermis of older |
Scientific name
Urocystis agropyri (G. Preuss) J. Schröth
Other scientific names
Urocystis tritici Körn.
Importance
High phytosanitary importance.
Significance
Flag smut is generally not an economically important disease, but, where present, yield losses can range from negligable to moderate levels (when susceptible cultivars are grown). Unless controlled, it can cause significant losses, mainly in autumn-sown wheat in regions with arid summers and mild winters.
Symptoms
Infected plants show chlorotic striping parallel to the veins of leaf blades. Leaf sheaths turn greyish-black due to the production of fungal sori. Masses of dark brown to black, powdery teliospores are produced in these narrow strips just beneath the epidermis. The epidermis then ruptures to release the teliospores. Diseased plants are often stunted and tiller profusely, and the spikes may not emerge. A severe infection usually induces the leaves to roll, producing an onion-type leaf appearance. Leaves become twisted and split lengthwise. The epidermis of older diseased plants tends to shred, releasing the teliospores.
Hosts
Bread wheats are the primary hosts of flag smut fungi, and the isolates attacking bread wheat tend to do so exclusively. There are few reports of flag smut on durum wheats and triticales.
U. agropyri attacks wheat, Aegilops crassa, Lolium temulentum and a number of other grasses.
Geographic distribution
Cosmopolitan but limited in the case of wheat . The disease is found in most winter wheat-growing areas and in cool, fall-sown spring wheat-growing areas.
Biology and transmission
Soilborne and externally seedborne. The spore balls consist of 1 to 4 dark teliospores (10-20 μm in diameter), surrounded by a layer of hyaline to brown sterile cells (total size 18-40 μm). These can survive in dry soil for more than a year.
Detection/indexing methods used
- At CIMMYT: Seed washing and filtration test.
- At ICARDA: Centrifuge wash test
Treatment/control
- Systemic fungicides such as carboxin or triadimenol preferred for seed treatment.
Procedures followed at the centers in case of positive test
- At CIMMYT: The seed lot is destroyed (the pathogen is quarantined in Mexico).
- At ICARDA: The seed lot is destroyed , the pathogen is quarantined in ICARDA Tel-Hadya station
References and further reading
Ballantyne B. 1996. Flag smut. In R.D. Wilcoxson and E.E. Saari (eds.), Bunt and Smut Diseases of Wheat: Concepts and Methods of Disease Management. Mexico, D.F.: CIMMYT. pp. 48-56.
Beniwal S, Karwasra SS, Chhabra ML. 1996. Evaluation of systemic fungicides against flag smut (Urocystis agropyri) of wheat. Annals of Applied Biology 128: 20-21.
CIMMYT. Wheat Doctor information sheet: Flag smut. [online] Available from URL: http://wheatdoctor.cimmyt.org/ Date accessed 06 April 2010
Diekmann M, Putter CAJ (eds.). 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14: Small Grain Temperate Cereals. Rome: Food and Agriculture Organization of the United Nations (FAO) and International Plant Genetic Resources Institute (IPGRI). p. 47.
Goel RK, Jhooty JS. 1985. Chemical control of flag smut of wheat caused by Urocystis agropyri. Indian Phytopathology 38: 749-751.
Line RF. 1993. Flag smut. In: Mathur SB, Cunfer BM. (eds.), Seed-borne Diseases and Seed Health Testing of Wheat. Copenhagen: Danish Government Institute of Seed Pathology for Developing Countries. pp. 53-57.
Loughman R. 1989 .Chemical control in Western Australia of soilborne flag smut caused by Urocystis agropyri. Australasian Plant Pathology 18(4): 94-95.
Oregon State University Extension Service. 2008. An online guide to plant disease control: Wheat (Triticum aestivum)—flag smut. [online] Available from URL: http://plant-disease.ippc.orst.edu/ Date accessed 06 April 2010
Prescott JM, Burnett PA, Saari EE, Ransom J, Bowman J, de Milliano W, Singh RP, Bekele G. 1986. Wheat Diseases and Pests: A Guide for Field Identification. Mexico, D.F.: CIMMYT. pp 16-17. [online] Available from URL: http://www.cimmyt.org/ Date accessed 06 April 2010
Wallwork H. 2000. Cereal Leaf and Stem Diseases. Adelaide: Grains Research and Development Corporation. pp. 24-25.
Wiese MV. 1987. Compendium of Wheat Diseases. 2nd edn. St. Paul, MN: APS Press. pp. 24-25.
|
Loose smut: The teliospores have been blown
Loose smut: The entire infloresence, except |
Scientific name
Ustilago tritici (Pers.) Rostr.
Other scientific names
Ustilago nuda var. tritici
Importance
Important in areas where seed treatment with appropriate systemic fungicides is not practiced.
Significance
Yield losses depend directly on the number of spikes affected by the disease; incidence is usually less than 1% and rarely exceeds 30% of the spikes in any given location.
Symptoms
Loose smut appears after the ear has emerged. The entire inflorescence, except the rachis, is replaced by masses of black teliospores. Initially these are covered by a membrane but this ruptures quickly to expose the smut. The spores are often blown away by the wind or washed off by rain leaving only the bare rachis and remnants of other floral structures. In some cultivars, infected plants may have dark green erect leaves with chlorotic streaks, which turn into necrotic streaks prior to the emergence of diseased ears.
Hosts
Wheat, triticale, Triticum dicoccum, T. monococcum, T. polonicum, T. spelta, T. turgidum.
Geographic distribution
Cosmopolitan
Biology and transmission
Ustilago tritica can be both seedborne and seed-transmitted. Wind-blown teliospores that land on the flowers of wheat plants germinate and infect the developing embryo of the kernel. The fungus survives as dormant mycelium in the embryo tissues of infected seed until the kernel germinates. The mycelium then develops along with the growing point of the plant. The fungus grows systemically in the host without causing symptoms. Spikelets are invaded intracellularly and all tissues except the rachis are converted to sori containing dry, dark–brown to black teliospores. Initially these spores are enclosed in a membrane; this quickly ruptures and teliospores are dispersed by wind and rain, traveling up to 150 m. Spores are deposited on flowering spikes and germinate to form promycelia, which fuse to produce infective hyphae. These hyphae penetrate the ovary wall and the fungus is established within the developing grain, completing the disease cycle. Thus, the floral parts of the spike are replaced with masses of black spores.
Infection occurs during wet, cloudy weather under cool to moderate temperatures (16-22°C). Within one week of flowering the ovary becomes resistant to infection
Infection and disease development are favored by cool, humid conditions, which prolong the flowering period of the host plant.
Detection/indexing methods used
- At CIMMYT: Seed washing and filtration test; Embryo test.
- At ICARDA: Embryo test
Treatment/control
- Systemic seed treatments such as carboxin, carboxin plus thiram, carbendazim, pyracarbolid, or terbutrazole.
Procedures followed at the centers in case of positive test
- At CIMMYT: Seed treatment with carboxin and captan.
- At ICARDA: Heavy infected seed lot is destroyed; valuable seed lot is treated and multiplied under controlled conditions
References and further reading
Agarwal VK, Chahal SS, Mathur SB. 1993. Loose smut. In Mathur SB, Cunfer BM (eds.), Seed-borne Diseases and Seed Health Testing of Wheat. Copenhagen: Danish Government Institute of Seed Pathology for Developing Countries. pp. 59-67.
CIMMYT. Wheat Doctor information sheet: Loose smut. [online] Available from URL: http://wheatdoctor.cimmyt.org/ Date accessed 06 April 2010
Diekmann M, Putter CAJ. (eds.). 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14: Small Grain Temperate Cereals. Rome: Food and Agriculture Organization of the United Nations (FAO) and International Plant Genetic Resources Institute (IPGRI). pp. 48-49.
Fuentes-Davila G, Goates BJ, Thomas P, Nielsen J, Ballantyne B. 2002. Smut diseases. In B.C. Curtis, S. Rajaram, and H. Gómez Macpherson (eds.), Bread Wheat: Improvement and Production. FAO Plant Production and Protection Series No. 30. Rome: Food and Agriculture Organization of the United Nations (FAO). Pp. 251-271. [online] Available from URL: http://www.fao.org/ Date accessed 06 April 2010
Nielsen J, Thomas P. 1996. Loose smut. In Burnett PA, Saari EE (eds.) Bunt and Smut Diseases of Wheat: Concepts and Methods of Disease Management. Mexico, D.F.: CIMMYT. pp 33-47.
Prescott JM, Burnett PA, Saari EE, Ransom J, Bowman J, de Milliano W, Singh RP, Bekele G. 1986. Wheat Diseases and Pests: A Guide for Field Identification. Mexico, D.F.: CIMMYT. pp. 14-15.
Rennie WJ. 1982. ISTA Handbook on Seed Health Testing. Section 2, Working Sheet No. 48: Wheat loose smut. Zürich: International Seed Testing Association.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 63.
Wiese MV. 1987. Compendium of Wheat Diseases. 2nd edn. St. Paul, MN: APS Press. p. 22.
Comments
- No comments found





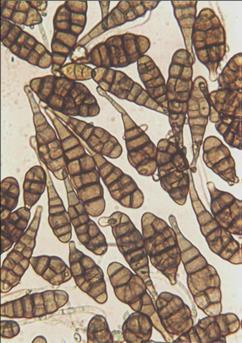



















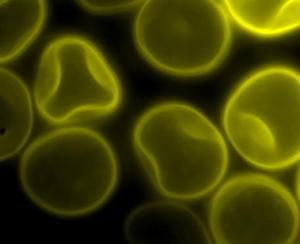
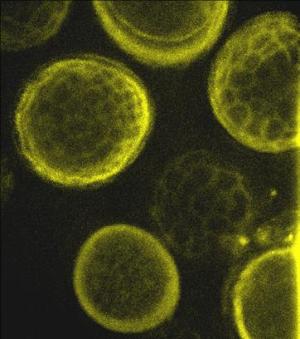



Leave your comments
Post comment as a guest