Fungi - cowpea
Contributors to this section: IITA, Nigeria (M. Ayodele, L. Kumar).
Scientific names
Colletotrichum lindemuthianum, Glomerella lindemuthiana Shear [teleomorph]
Other scientific names
Gloeosporium lindemuthianum Sacc., Gloeosporium socium Sacc.
Importance
High
Significance
Anthracnose affects yield, seed quality and marketability of the crop. The disease causes huge losses in temperate and subtropical zones. Losses of 35,925 tonnes due to anthracnose have been estimated in Rwanda. (Tu, 1988). Yield losses of 95% have been recorded in Colombia and over 92% in Malawi (Allen, 1983). In East Africa, anthracnose is important in Kenya, Uganda and Tanzania. It is recurrent in the Great Lakes Region of Rwanda, Burundi and the Kivu Province of Zaire (CIAT, 1981).
In South America, it had been reported that C. lindemuthianum caused severe damage in Brazil (Vieira, 1983), Argentina (Ploper, 1983), Mexico (Crispin-Medina and Campos-Avila, 1976), Guatemala, Costa Rica, Nicaragua (Echandi, 1976), Peru, Ecuador, and Colombia (Olarte et al., 1981.
Symptoms
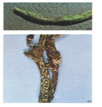
The fungus infects all stages of the plant, flowering , podding, pre-emergence, seedling and vegetative growing stages and all plant parts including the pods, leaves and seeds. Initial symptoms may appear on cotyledonary leaves as small, dark brown to black lesions.The infected tissues manifest minute rust-coloured specks. The specks gradually enlarge longitudinally and form sunken lesions or eye-spots.
Leaves: lesions first develop on leaf petioles, the lower surface of leaves and leaf veins as small, angular, brick-red to purple spots which become dark brown to black. Later, the lesions may also appear on veinlets on the upper surface of leaves.
Seedlings: lesions enlarge on the hypocotyl of the young seedling, causing rot.
Stem: eye-shaped lesion develop.
Pod: infections appear as rusty brown spots with small, brown specks, sunken cankers delimited by a slightly raised black ring and surrounded by a reddish-brown border. young pods shrivel and dry up.
Seed: discolouration, dark brown to black cankers, brown to light chocolate spots on the seed coats.
 C. lindemuthianum (photo: IITA) |
Hosts
The major hosts infected by this fungus are Vigna unguiculata (cowpea), Cajanus cajan (pigeon pea), Lablab purpureus (hyacinth bean), Phaseolus (beans), Phaseolus vulgaris (common bean), Vigna sinensis ssp. sesquipedalis (asparagus bean.
The minor hosts include Glycine max (soyabean), Lens culinaris ssp. culinaris (lentil), Phaseolus coccineus (runner bean), Pisum sativum (pea), Vicia faba (broad bean), Vigna mungo (black gram), Vigna radiata (mung bean), and Canavalia ensiformis (gotani bean.
Geographic distribution
Worldwide
Biology and transmission
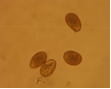
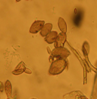
Conidia which are round or elongated are borne on acervuli which may be present on pods, leaves, stems and branches. The mycelium is hyaline, branched and septate.
A conidium takes 6-9 hours to germinate under favourable environmental conditions. The pathogen penetrates the cuticle and epidermis mechanically (Leach, 1923). Following penetration of host cells, when temperatures are favorable, infectious hyphae enlarge and grow between the cell wall and protoplast for 2-4 days without apparent damage to host cells ).
C. lindemuthianum is seed borne and seed transmitted.
C. Lindemuthianum has various strains classified on the basis of host reaction.Two distinct races have been characterized as alpha and beta. Several new races have been identified in Canada, USA, Europe, Brazil and Africa.
Mordue, (1971a,b) reported that the fungus can survive for at least 2 years in seed. The longevity in infected pods and seeds varies considerably depending on environmental conditions. The pathogen was able to survive for at least 5 years on pods and seeds that were air-dried and kept in storage at 4°C or on dry, infected plant materials left in the field in sealed polyethylene envelopes (Tu, 1983).
C. lindemuthianum survives as dormant mycelium within the seed coat, sometimes even within cells of cotyledons, as spores between cotyledons or elsewhere in the seed (Zaumeyer and Meiners, 1975). The fungus survives in the seed as long as the seed remains viable It also survives in infected crop residues.
Infection is favoured by moderate temperatures between 13 and 26°C; (Ferrante and Bisiach, 1976), while Tu and Aylesworth, (1980) reported that infection is favoured by an optimum temparature of 17-24°C.
Humidity of more than 92% or free moisture is required during all stages of conidium germination, incubation and subsequent sporulation; (Tu, 1982).
Zaumeyer and Thomas, (1957) reported that the dissemination and spread of the conidia, and the development of severe anthracnose epidemics is favoured by wind or rain. C. lindemuthianum required about 10 mm of rain to establish infection (Tu, 1981). Conidia spread may be dispersed within the crop by insects, animals and man, especially when foliage is moist (Zaumeyer and Thomas, 1957).
Detection/indexing methods used at IITA
- Agar method. The Nutrient Broth Yeast extract (NBY) agar medium is used for the detection of the pathogen
- According to ISTA randomly select a sub sample of 500 seeds (or less if fewer seeds are available) from the seed lot.
- Surface disinfection of seeds using 10% Sodium hypochlorite for 3 minutes.
- Rinse the seed in sterile distilled water and blot off excess.
- Plate the material on NBY agar medium and incubate at 28oC for 4days.
- Examine plate under stereo microscope.
- Make microscopic slides of fungal fruiting bodies observed in growth.
- Examine under compound microscope to identify the fungal fruiting bodies and spores isolated from the mycelial growth
- Subculture on NBY to obtain pure cultures of the pathogen for pathogenicity tests/ preservation
- Make microscopic slides of the spores and re examine under the Compound microscope for confirmation and purity.
Treatment/cControl
- Seed treatment with mancozeb(Ethylene Bisdithiocarbamate ) 80g a.i./kg of seeds
- Plant pathogen free healthy resistant varieties. IITA has bred several resistant lines
- Production of seeds for export in Certified Pest Free areas (PFA)
- Fungicidal field sprays in the field during active growth
Procedures in case of positive test at IITA
- Seeds from lines testing positive are treated with mancozeb 80g/kg of seeds. The treated seeds are retested after 3 days. If the pathogen is isolated from the treated lines, the lines are rejected. Not for international distribution in compliance to the importing countries’ phytosanitary regulations.
References and further reading
Allen DJ. 1983. The pathology of tropical food legumes: disease resistance in crop improvement. Chichester, UK: John Wiley & Sons
CAB International. 2007. Crop Protection Compendium, 2007 Edition. Wallingford, UK: CAB International
CIAT. 1981. Potential for field beans in eastern Africa: proceedings of a regional workshop held in Lilongwe, Malawi, 9-14 March 1980. CIAT Series 03EB-1. Cali, Colombia: CIAT.
Crispin A, Campos J. 1976. Bean diseases of importance in Mexico in 1975. Plant Disease Reporter, 60(6):534-535.
Echandi E. 1976. Principal fungus diseases of bean (Phaseolus vulgaris) in the American tropics in different ecological zones. Fitopatologia Brasileira, 1(3):171-177
Ferrante GM, Bisiach M. 1976. Comparison of methods for experimental infection of bean with Colletotrichum lindemuthianum. Rivista di Patologia Vegetale, IV, 12(3/4):99-118;
Leach JG. 1923. The parasitism of Colletotrichum lindemuthianum. Minnesota Agricultural Experiment Station Technical Bulletin, 14.
Mordue JEM. 1971a. Colletotrichum lindemuthianum. Descriptions of Pathogenic Fungi and Bacteria Set 32, Sheet No. 316. Wallingford, UK: CAB International.
Mordue JEM. 1971. Glomerella cingulata. CMI Descriptions of Pathogenic Fungi & Bacteria No. 315. Wallingford, UK: CAB International.
Olarte MD, Osorio G, Puerta OD, Isaza L. 1981. Mechanisms for primary infection by anthracnose (Colletotrichum lindemuthianum) on bean (Phaseolus vulgaris) in Eastern Antioch. Fitopatologia Colombiana, 10(1/2):23-28;
Ploper LD. 1983. Bean diseases in Northwest Argentina and their control. Publicación Miscelánea Estación Experimental Agro-Industrial "Obispo Colombres" de Tucumán, No.74:87-103;
Olarte MD, Osorio G, Puerta OD, Isaza L. 1981. Mechanisms for primary infection by anthracnose (Colletotrichum lindemuthianum) on bean (Phaseolus vulgaris) in Eastern Antioch. Fitopatologia Colombiana, 10(1/2):23-28;
Tu JC. 1981. Anthracnose (Colletotrichum lindemuthianum) on white bean (Phaseolus vulgaris L.) in southern Ontario: spread of the disease from an infection focus. Plant Disease, 65(6):477-480
Tu JC. 1982. Effect of temperature on incidence and severity of anthracnose on white bean. Plant Disease, 66(9):781-783;
Tu JC. 1983. Epidemiology of anthracnose caused by Colletotrichum lindemuthianum on white bean (Phaseolus vulgaris) in southern Ontario: survival of the pathogen. Plant Disease, 67(4):402-404
Tu JC. 1988. Control of bean anthracnose caused by the delta and lambda races of Colletotrichum lindemuthianum in Canada. Plant Disease, 72(1):5-8.
Tu JC, Aylesworth JW. 1980. An effective method of screening white (pea) bean seedlings (Phaseolus vulgaris L.) for resistance to Colletotrichum lindemuthianum. Phytopathologische Zeitschrift, 99(2):131-137;
Zaumeyer WJ, Meiners JP. 1975. Disease resistance in beans. Annual Review of Phytopathology, 13:313-334.
Zaumeyer WJ, Thomas HR. 1957. A monographic study of bean diseases and methods for their control. United States Department of Agricultural Technical Bulletin, 868
Cercospora leaf spots, Leaf spot of cowpea
Scientific name
Cercospora canescens
Other scientific name
Cercospora vignicaulis Tehon
Importance
High
Significance
Yield loss due to seed infection has not been quantified
Symptoms
The symptoms are prominent on the leaves alone. However, the fungus has been isolated from infected seeds which are symptomless. Symptoms found on various plant parts are as follows:
Leaves: subcircular to broadly irregular spots having pale tan to grey centre surrounded by dark brown or reddish margin. The spots coalesce to form round lesions which are brown and necrotic with dark, and slightly depressed edges.
Pods: damaged pods, drying up.
Stem: lesions on the stem, and cotyledons
Hosts
Although the disease occurs mainly on cowpeas and on grain legumes, other major and minor hosts have been identified.
The major hosts are Vigna unguiculata (cowpea), Amaranthus (grain amaranth), Glycine max( soybean) , Lablab purpureus (hyacinth bean), Lycopersicon esculentum (tomato), Phaseolus (beans), Ricinus , Vicia (vetch), Vigna (cowpea), Voandzeia subterranea (bambara groundnut).
Other hosts obtained from artificial inoculations are :
Crotalaria juncea (sunn hemp), Psophocarpus tetragonolobus, (winged bean), Vigna angularis (adzuki bean), Vigna mungo (black gram) and Vigna radiata (mung bean).
Geographic distribution
The disease is widespread in warmer subtropical and tropical regions. The fungus has been reported in the Eastern region of USA (Farr et al., 1989); Bangladesh, China, India, Indonesia, Thailand, Africa, Brazil and Samoa.
Biology and transmission
Abundant fruiting bodies on the lower surface of the leaf. The conidia are uniform in colour, pale to medium brown, multiseptate, medium to large size, conidial scar present on the rounded apex, thickened hilum. Most conidia are formed at 28°C, while at 24°C and 32°C less conidia are formed. The presence of light increases the number of conidia (Mulder and Holliday, 1975).
Detection/indexing methods used at IITA
Blotter method.
Procedure
According to ISTA, randomly select a sub sample of 500 seeds (or less if fewer seeds are available) from the seed lot.
- Surface disinfection of seeds using 10% Sodium hypochlorite for 3 minutes.
- Rinse the seed in sterile distilled water and blot off excess.
- Plate the material on blotter and incubate at 28oC for 4days.
- Examine plate under stereo microscope.
- Make microscopic slides of fungal fruiting bodies observed in growth.
- Examine under compound microscope to identify the fungal fruiting bodies and spores
- isolated from the mycelial growth
- Subculture on NBY to obtain pure cultures of the pathogen .
- Make microscopic slides of the spores and re examine under the compound microscope
- for confirmation and purity. Pick single spores and transfer unto V8 agar for sporulation
(The V8 juice agar contains: 3.0 g of calcium carbonate (CaCO3),2.5 g of glucose 20 g of agar powder, 200 ml of V8 juice adjust to 1 litre and autoclave, cool to about 400C. Add 1g of streptomycin powder to prevent bacterial growth).
- Incubate for 2 days at 27oC. Reexamine for purity. For preservation subculture unto ¼ strength PDA slants. Incubation beyond 3 days causes the spores to collapse.
Treatment/control
- Seed treatment with mancozeb ( Ethylene Bisdithiocarbamate ) 80g/kg of seeds
- Plant pathogen free healthy resistant varieties. IITA has bred several resistant lines.
- Production of seeds for export in Certified Pest Free areas(PFA)
- Fungicidal field sprays in the field during active growth
Procedures in case of positive test at IITA
- Seed treatment with mancozeb 80g/kg of seeds. The treated lines to be retested after 3 days of treatment. If the pathogen is isolated from the treated lines, the lines are rejected. Not for international distribution in compliance to the importing countries’ phytosanitary regulations.
References and further reading
CAB International. 2007. Crop Protection Compendium, 2007 Edition. Wallingford, UK: CAB International.
Farr DF, Bills GF, Chamuris GP, Rossman AY. 1989. Fungi on plants and plant products in the United States. St. Paul, Minnesota, USA: APS Press
Mulder JL, Holliday P. 1975. Cercospora canescens. CMI Descriptions of Pathogenic Fungi and Bacteria, No. 462. Wallingford, UK: CAB International.
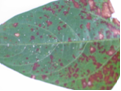
Field symptom (photo:IITA) |

growth on agar (photo:IITA) |
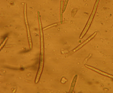
conidia (photo:IITA) |
Scientific name
Cercospora cruenta; Mycosphaerella cruenta Latham [Teleomorph].
Other scientific name
Pseudocercospora cruenta (Sacc.) Deighton
Importance
High
Significance
Fery et al., (1977) reported that M. cruenta reduced the number of pods per plant and the number of seeds per pod. This Cercospora leaf spot disease has been reported by Williams, (1977) to have caused considerable yield losses in cowpea fields in Nigeria.
Schwartz and Pastor-Corrales, (1989) in a study in USA, reported that M. cruenta leaf spot of cowpea reduced the seed yield of the susceptible cv. Colossus by 35.6% . While in Varanasi, India, leaf spot caused by M. cruenta was found to cause serious disease in lobiya (Vigna unguiculata) (Pant, 1989).
Symptoms
The fungus infects the pods, leaves and stems showing various symptoms such as:
Leaves: brown or rust-coloured circular to angular spots, coalesce forming lesions, chlorosis, abnormal leaf fall, and fungal growth on the leaves
Stems: Lesions, discoloration on branches,
Pods: lesions, discoloration
Seed: poor to no germination,small and abnormal
Whole plant: abnormal growth and development.
Hosts
The major hosts for this pathogen are Vigna unguiculata (cowpea), Phaseolus (beans), ) while some recorded minor hosts are Calopogonium, Lablab purpureus (hyacinth bean), Mucuna (velvetbeans), and Mucuna pruriens (Buffalobean).
Geographic distribution
Cosmopolitan
Biology and transmission
The perfect stage produce colourless, 1-septate, ascospores with upper cell which sometimes are slightly larger than the lower cell, straight to slightly curved,
Microconidia are rod-shaped, hyaline, aseptate, produced in pycnidia in or near lesions formed by the imperfect stage, Cercospora. The conidia are thin-walled, filiform, smooth, and hyaline to olivaceous-brown, 4-9-septate. M. cruenta survives between growing seasons on crop residues, diseased leaf and stem crop residues of cowpea (Vigna unguiculata).
The disease is seed borne and seed transmitted
Detection/indexing methods used at IITA
Blotter method.
Procedure
- According to ISTA, randomly select a sub sample of 500 seeds (or less if fewer seeds are available) from the seed lot.
- Surface disinfection of seeds using 10% Sodium hypochlorite for 3 minutes.
- Rinse the seed in sterile distilled water and blot off excess.
- Plate the material on blotter and incubate at 28oC for 4days.
- Examine plate under stereo microscope.
- Make microscopic slides of fungal fruiting bodies observed in growth.
- Examine under compound microscope to identify the fungal fruiting bodies and spores isolated from the mycelial growth.
- Subculture on NBY to obtain pure cultures of the pathogen .
- Make microscopic slides of the spores and re examine under the compound microscope for confirmation and purity. Pick single spores and transfer unto V8 agar for sporulation
- Incubate for 2 days at 27oC. Reexamine for purity. For preservation subculture unto ¼ strength PDA slants
Treatment/control
- Use resistant varieties available in IITA
- Seed treatment with mancozeb( Ethylene Bisdithiocarbamate ) 80g/kg of seeds
- Plant pathogen free healthy resistant varieties.
- Production of seeds for export in Certified Pest Free areas(PFA)
- Fungicidal field sprays in the field during active growth.
Procedures in case of positive test at IITA
- Seed treatment with mancozeb 80g/kg of seeds. The treated lines to be retested after 3 days of treatment. If the pathogen is isolated from the treated lines, the lines are rejected. Not for international distribution in compliance to the importing countries’ phytosanitary regulations.
References
CAB International. 2007. Crop Protection Compendium, 2007 Edition. Wallingford, UK: CAB International
Fery RL, Dukes PD. 1977a. Cercospora leaf spot of southernpea: studies on yield-loss and genetics of resistance. HortScience, 12(3):234.
Pant DC. 1989. Perpetuation of leaf spot organism of lobiya. Indian Phytopathology, 42(1):187-188.
Schwartz HF, Pastor-Corrales MA. 1989. Bean production problems in the tropics, 2nd edition. Cali, Colombia: CIAT
Williams RJ. 1977. Identification of multiple disease resistance in cowpea. Tropical Agriculture, 54(1):53-59.
 |
Scientific name
Fusarium oxysporum f.sp. tracheiphilum (E.F.Sm.) Snyder & H.N. Hansen
Other scientific names
Fusarium bulbigenum var. tracheiphilum, E.F. Sm. Wollenw
Fusarium tracheiphilum E.F. Sm.
Fusarium bulbigenum Cooke & Massee
Importance
High
Significance
Toler et al. (1963) reported that Fusarium wilt of cowpea caused by F. oxysporum f.sp. tracheiphilum is a destructive disease in the Southern and Eastern USA . In India, the yield loss caused by the fungus was 26.8-64.5% in 1954 (Singh, 1954) and 74.6% in 1955 (Singh and Sinha, 1955). And in 1996, the wilt incidence in India was recorded as 30% by Ushamalini (1996).
Symptoms
Fusarium oxysporum f. sp. tracheiphilum attacks young and old plants. Symptoms of the disease are visible on leaves, stem and roots. The vascular bundles show brownish-black discoloration. All plant parts are affected and showing different symptoms such as on:
Leaves: yellowing, withering, Chlorosis, leaf drooping,
Shoots: dry and naked
Stem: blackened and swollen, wilt (Singh and Sinha, 1955
Pods: presence of pinkish-white fungal growth
Seeds: discoloration, shrivelling, ashy white and shrunken (Ushamalini, 1996)
Roots: rot; reduced root system; absence of lateral roots , lesions
Whole plant: systemic infection, colonization of xylem vessels resulting in chlorosis, die back, dwarfing.
Hosts
The major hosts of this pathogen are:
Vigna unguiculata (cowpea) and some minor hosts such as Chrysanthemum vestitum Glycine max (soyabean), and Phaseolus vulgaris (common bean).
Geographic distribution
Cosmopolitan
Biology and transmission
Morphology of F. oxysporum f.sp. tracheiphilum on oat meal agar, has a cotton wool appearance, with a white to purple aerial mycelium. The microconidia are ellipsoidal, hyaline, and aseptate . The macroconidia are slightly curved, mostly septate. The chlamydospores are ellipsoidal to globose, terminal or intercalary and aseptate. The fungus also produces small, abundant sporodochia on PDA in 8-12 days at 26-30°C.The symptoms of vascular wilt are severe at higher temperatures of 27°C (Swanson and Van Gundy, 1985). Swanson, (1984). Harris and Ferris, (1991b) further reported that the wilt symptom is very severe when the fungus is in association with the nematode , Meloidogyne javanica . F. oxysporum f. sp. tracheiphilum is seed borne and seed transmitted. Seeds from wilted plants stored in the refrigerator for 4 years remained viable (Armstrong and Armstrong, 1950). The fungus was found to be present in the seed coat, cotyledon, and embryo.
Detection/indexing methods used at IITA
Agar method
Procedure
- Randomly select a sub sample of 500 seeds (or less if fewer seeds are available) from the seed lot. Surface disinfection of seeds using 10% Sodium hypochlorite for 3 minutes.
- Rince the seed in sterile distilled water and blot off excess.
- Plate the material on NBY agar medium and incubate at 28oC for 4days.
- Examine plate under stereo microscope.
- Make microscopic slides of fungal fruiting bodies observed in growth.
- Examine under compound microscope to identify the fungal fruiting bodies and spores isolated from the mycelial growth
- Subculture on PDA to obtain pure cultures of the pathogen for pathogenicity tests/ preservation
- Make microscopic slides of the spores and re examine under the Compound microscope for confirmation and purity.
Treatment/control
- Use resistant varieties. Available at IITA.
- Seed treatment with mancozeb( Ethylene Bisdithiocarbamate ) 80g/kg of seeds
- Plant pathogen free healthy resistant varieties.
- Production of seeds for export in Certified Pest Free areas(PFA)
- Growing on test in the screen house, active growth inspection, wilted plants rogued and incinerated
Procedures in case of positive test at IITA
- Seed treatment with mancozeb 80g/kg of seeds. The treated lines to be retested after 3 days of treatment. If the pathogen is isolated from the treated lines, the lines are rejected. Not for international distribution in compliance to the importing countries’ phytosanitary regulations.
For import or export:
- For valuable germplasm material to be used for crossing by breeders, growing on test in the containment , active growth inspection, wilted plants rogued and incinerated
- Seeds from symptomless plants harvested and retested before release for use.
References and further reading
Armstrong GM, Armstrong JK. 1950. Biological races of the Fusarium causing wilt of cowpeas and soybeans. Phytopathology, 40:181-193
Harris AR, Ferris H. 1991. Interactions between Fusarium oxysporum f.sp. tracheiphilum and Meloidogyne spp. in Vigna unguiculata. 1. Effects of different inoculum densities on Fusarium wilt. Plant Pathology, 40(3):445-456.
ISTA. 1985. International rules for seed testing. Seed Science and Technology, 13:484-487.
Singh RS, Sinha RP. 1955. Studies on the wilt disease of cowpea in Uttar Pradesh. J. Indian Bot. Soc., 34:375-381.
Swanson TA. 1984. Root-knot nematode and Fusarium wilt diseases of cowpea and soybean. PhD. thesis University of California, Riverside.
Swanson TA, Gundy SDVan. 1985. Influences of temperature and plant age on differentiation of races of Fusarium oxysporum f.sp. tracheiphilum on cowpea. Plant Disease, 69(9):779-781;
Thomason IJ, Erwin DC, Garder MJ. 1959. The relationship of the root-knot nematode, Meloidogyne javanica to Fusarium Wilt of Cowpea. Phytopathology, 49:602-606.
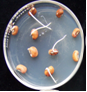
clean seeds (photo:IITA) |

infected seeds (photo:IITA) |
growth on agar (photo:IITA) |
conidia (photo:IITA) |
Scientific name
Sphaceloma sp.
Other scientific names
Elsinoe phaseoli,Jenkins ; Elsinoe vignicola
Importance
High
Significance
High.
Serious disease causing loss of foliage, pods and seeds. In severe case there is complete loss of yield. Exact yield loss caused by this fungus has not been quantified.
The disease is endemic in Zaria, Northern Nigeria.
Symptoms
The fungus attacks the stem, leaves, and pods of Vigna unguiculata
Symptoms on the different parts such as on :
Leaves: spots on both leaf surfaces, cupped. Appearance of small grayish scab lesion along the veins. Leaf distortion, Ragged appearance
Stems: oval to elongated silver grey lesions surrounded by red or brown elliptical ring. Lesions coalesce, distortion
Pod: sunkened spots with grey centers surrounded by brown borders , malformation, dark coloured pycnidia formed in the brown spots.
Hosts
Vigna unguiculata, ( Cowpea) and Lima beans, Beans.
Geographic distribution
The disease has been reported in West and East Africa, Central America and severe outbreaks have been reported in Surinam ( Singh, S R and Allen D. J (1979).
Biology and transmission
The mycelium is hyaline, scanty and submerged. Conidia are hyaline to pale coloured. The conidia are produced in pycnidia. The ascospores borne on the asci are hyaline, pale colored oblong to elliptical, and 3 septate.
Detection/indexing methods used at IITA
- Collect infected plant parts ( stem, leaf, pods and seeds) from the field
- Prepare PDA+streptomycin and rose bengal
- Cowpea Pod Agar amended with PDA adding Streptomycin sulphate (1.5g l-1)
- And Rose Bengal (0.0025g l-1)
- Cut the different infected parts into small portions
- Surface sterilize in 10% sodium hypochlorite for 3 minutes
- Blot with sterile paper towel
- Place five portions in each plate on the medium
- Incubate the plate at 27c for 5-7 days
- Examine under microscope.
Treatment/control
- Use resistant varieties. Available at IITA.
- Seed treatment with mancozeb( Ethylene Bisdithiocarbamate ) 80g/kg of seeds
- Plant pathogen free healthy resistant varieties.
- Production of seeds for export in Certified Pest Free areas(PFA)
Procedures in case of positive test at IITA
- Seed treatment with mancozeb 80g/kg of seeds. The treated lines to be retested after 3 days. If the pathogen is isolated from the treated lines, the lines are rejected. Not for international distribution in compliance to the importing countries’ phytosanitary regulations
- For import or export:
- For valuable germplasm material to be used for crossing, growing on test in the containment , active growth inspection is conducted. Plants with scab symptoms are rogued and incinerated
- Seeds from symptomless plants harvested and retested for freedom from fungus.
References and further reading
Singh SR, Allen DJ. 1979. Cowpea pests and Diseases. IITA monograph. Manual series No 2. Trop. Grain Legume Entomology, IITA, Ibadan, Nigerial.
|
Pod and stem scab (photo:IITA) |
Pod and stem scab (photo:IITA) |
Stem scab (photo:IITA) |
Brown blotch, Brown blotch of cowpea
Scientific name
Colletotrichum truncatum(Schwein.) Andrus & W.D. Moore, 1934
Other scientific names
Colletotrichum dematium f. truncatum, (Schwein.) Arx
Vermicularia truncata Schwein. 1832
Importance
High
Significance
Rheenen, (1975) attributed yield losses of 30% to anthracnose infection in Nigeria. A survey conducted in two states in Brazil detected the disease in 57% of the fields (Lehman et al., 1976). Anthracnose of maturing plants causes serious losses, particularly during the rainy period when shaded lower branches and leaves die due to severe infection.
Symptoms
C.truncatum affects all plant stages and parts - flowering , podding, seedling stages also the pods, inflorescence, leaves, stems and whole plant. The stems, pods and leaves may be infected without showing symptoms. In the advanced stages of anthracnose in the late reproductive stages, infected tissues are covered with black fruiting bodies (conidiomata) which produce setae (minute black spines).
Symptoms found on the different plant parts such as on the:
- Inflorescence: lesions.
- >Leaves: lesions; abnormal colours and forms; fungal growth, necrosis of laminar veins, leaf rolling and petiole cankers. Premature defoliation, girdling of the leaf and petiole Developpment of shepherd's crook .
- Seedlings: discoloration; canker, premature death, and severe reductions in seedling emergence was recorded by Athow, (1987).
- Pods: lesions. irregularly-shaped, brown areas , blanking( no pod formation), reduction in pod size and number.
- Seeds: small, irregular, grey areas with black specks, brown staining, symptomless. The fungus is confined at first to the seed coat. The infected seeds may die during germination or, if they germinate, produce infected seedlings.
- Whole plant: damping off; dwarfing, early senescens.
Hosts
C. truncatum has a wide host range among the local edible legumes. The fungus attacks crops considered as major and minor hosts. The major hosts are Vigna unguiculata (cowpea), Arachis hypogaea (groundnut), Cajanus cajan (pigeon pea), Capsicum annuum (bell pepper), Centrosema , Centrosema pubescens (Centro), Glycine max (soyabean), Medicago sativa (lucerne), Phaseolus (beans), Phaseolus lunatus (lima bean), Phaseolus vulgaris (common bean), Pisum sativum (pea), Vigna (cowpea), Vigna mungo (black gram), Vigna radiata (mung bean).
In addition to the host species mentioned above, C. truncatum has been isolated from several weed species (Hartman et al., 1986; Roy, 1982).
Geographic distribution
Cosmopolitan
Biology and transmission
The hyphae are hyaline, branched and septate
C. truncatum has crowded, black acervuli which are borne on well-developed stromata. It produces numerous, black mixed setae in culture, some are long and others short.
The conidia are borne singly on conidiophores, bluntly tapered, curved, unicellular, and hyaline. Conidia produce one or two short germ tubes which produce dark, sticky appressoria when in contact with the surface of the host plant
The disease is seed borne and seed transmitted .
Detection/indexing methods used at IITA
Seed Health Tests
- Agar method The Nutrient Broth Yeast extract (NBY) agar medium is used for the detection of the pathogen.
Procedure
- Randomly select a sub sample of 500 seeds (or less if fewer seeds are available) from the seed lot.
- Surface disinfection of seeds using 10% Sodium hypochlorite for 3 minutes.
- Rince the seed in sterile distilled water and blot off excess.
- Plate the material on NBY agar medium/ Blotter and incubate at 28oC for 4days.
- Examine plate under stereo microscope.
- Make microscopic slides of fungal fruiting bodies observed in growth.
- Examine under compound microscope to identify the fungal fruiting bodies and spores isolated from the mycelial growth
- Subculture on NBY to obtain pure cultures of the pathogen for pathogenicity / preservation
- Make microscopic slides of the spores and re examine under the compound microscope for confirmation and purity.
Treatment/control
- Seed Treatments with mancozeb followed by 2 foliar sprays of mancozeb.
Procedures in case of positive test at IITA
- Seed treatment with mancozeb 80g/kg of seeds. The treated lines to be retested after 3 days. If the pathogen is isolated from the treated lines, the lines are rejected. Not for international distribution in compliance to the importing countries’ phytosanitary regulations
- For import or export: for valuable germplasm material to be used for crossing, growing on test in the containment , active growth inspection is conducted. Plants with anthracnose symptoms are rogued and incinerated
- Seeds from symptomless plants harvested and retested
References and further reading
Athow KL. 1987. Fungal diseases. In: Wilcox JR, editor. Soybeans: Improvement, Production and Uses, 2nd edition, Monogr. 16. Madison, USA: American Society of Agronomy, 687-727.
Hartman GL, Manandhar JB, Sinclair JB. 1986. Incidence of Colletotrichum spp. on soybeans and weeds in Illinois and pathogenicity of Colletotrichum truncatum.. Plant Disease, 70(8):780-782
Lehman PS, Machado CC, Tarrago MT. 1976. Frequency and severity of soybean diseases in the States of Rio Grande do Sul and Santa Catarina. Fitopatologia Brasileira, 1(3):183-193
Rheenen HA. 1975. Soybeans in the northern states of Nigeria. In: Luse RA, Rachie KO, editors. Proceedings of IITA Collaborators Meeting on Grain Legume Improvement. Ibadan, Nigeria: IITA, 158-159.

Pod symptom (photo:IITA) |
Growth on agar (photo:IITA) |
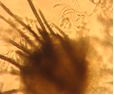
Conidia and setae (photo:IITA) |
Clean seeds (photo:IITA) |

Infected seeds (photo:IITA) |

Conidia |
Scientific name
Uromyces appendiculatus(Pers.) Unger (1816)
Other scientific names
Uromyces appendiculatus, Uromyces phaseoli,
Uromyces phaseolorum (DC.) de Bary,
Puccinia phaseoli-trilobi Schwein (1834),
Uromyces vignae-luteolae Henn.(1907).
Importance
High
Significance
Infection at the early stages of plant growth , results to crop failure. Yield losses reaching 28-54, 8-33 and 13-29% on different cultivars were reported by Gonzalez and Garcia (1996).
Symptoms
All plant parts above the ground are susceptible to infection. Infection appears at all stages of the plant growth.
- On the Leaves: yellow to yellowish-brown small raised blister like spots, as infection progresses, the blisters erupt and powder like uredo spores are exposed. The spores reinfect the plant and defoliation resulting to death of the plant.
- Stem: brown spots, spores and hyphae are borne internally and externally, spores visible to the eye
- Pods: brown spots, decrease in the number of pods per plant.
- Seed: malformation, decrease in weight of seed , sometimes symptomless
Hosts
The major hosts for the rust fungi are Vigna unguiculata (cowpea), Cajanus cajan (pigeon pea), Glycine max (soyabean), Lablab purpureus (hyacinth bean), Phaseolus (beans), Phaseolus lunatus (lima bean), Phaseolus vulgaris (common bean), Vigna angularis (adzuki bean), Vigna mungo (black gram) and some recorded minor hosts are Phaseolus coccineus (runner bean), Vigna radiata (mung bean), Vigna umbellata (Rice- bean), Voandzeia subterranea (bambara groundnut.
Geographic distribution
Rust is a universal disease of beans and is world wide in spread and distribution.
Biology and transmission
The mycelium is hyaline, branched and septate existing internally in host tissue producing various sori . It is autoecious having white pycnidia in small groups. Aecia are very rare but the aeciospores are globose to ellipsoid. Uredinia , sometimes absent but if present are pale brown, solitary or sometimes aggregated, minute,and cinnamon brown. Urediniospores are globose to subglobose.
Telia are blackish-brown to black. The teliospores are subglobose to ovoid or ellipsoid and rounded at the apex.
There can be crop failure if infection takes place in the early stages of plant growth. Overcast conditions and a temperature of 20-25ºC favour the pathogen. Twenty races of the pathogen were reported by Staveley (1984a) when he used 19 different bean cultivars in an experiment in USA.
Detection/indexing methods used at IITA .
- Two methods are used for isolation of the pathogen. Seed washing method and leaf isolation.
Seed washing method
- Randomly select a sub sample of 500 seeds (or less if fewer seeds are available) from the seed lot and place in a 250-ml flask.
- Add 100 ml of water and 5 drops of tween 80. Shake vigorously for 30 sec.
- Pour 10 ml of the resulting suspension into a test tube and centrifuge in table-top centrifuge for 5 minutes at 4000 rpm.
- Pour off the supernatant and resuspend the pellet in the bit of remaining water by tapping the tube with your finger. Place of a drop of the suspension on a microscope slide, cover with a cover slip, and inspect the entire slide at X10 and above under the compound microscope.
- Look for spores, fruiting bodies. Identify
Leaf isolation method/Slide
- Collect infected leaf sample from the field.
- Harvest Uromyces appendiculatus spores from infected samples using a needle on to a clean slide.
- Add a drop of lactophenol or cotton blue
- View under microscope to check for uredospores
Treatment/control
- Seed treatment with mancozeb followed by field spray before flowering reduced infection but did not eradicate the pathogen
- Plant resistant varieties
- Use disease free seeds and multiply seeds in PFAs
Procedures in case of positive test
- Discard. Not for international distribution in compliance to the importing countries’ phytosanitary regulations
- For import or export: For important germplasm material to be used for crossing, growing on test in the containment , active growth inspection, plants with rust symptoms are rogued and incinerated
- Seeds from symptomless plants harvested and retested
References and further reading
Gonzalez M, Garcia E. 1996. Evaluation of losses due to the rust on bean (Phaseolus vulgaris L.) in four sowing times in Cuba. Agronomia Mesoamericana, 7:95-98.
Stavely JR. 1984. Genetics of resistance to Uromyces phaseoli in a Phaseolus vulgaris line resistant to most races of the pathogen. Phytopathology, 74(3):339-344;
Staveley JR. 1984b. Pathogenic specialization in Uromyces phaseoli in the United States and rust resistance in beans. Plant Disease, 68:95-99.
|
Infected leaf (photo:IITA) |
Infected leaf (photo:IITA) |
Uredospores (photo:IITA) |
Leaf smut, Black spot of pulses
Scientific name
Entyloma vignae
Other scientific name
Protomycopsis phaseoli
Importance
High
Significance
The disease is of economic importance in Brazil where yield losses of 30-40% were reported.
Symptoms
The symptoms are very prominent on the leaves.
Leaves: dark ash grey to black, circular, purplish spots surrounded by yellow hallow border. As infection progresses, spots coalesce, become dark purple leaves become ragged and fall off.
Whole plant: severe infection causes defoliation
Hosts
The only listed hosts are Vigna unguiculata ,(cowpea), Lablab purpureus ,and (hyacinth bean)Vigna radiata (mung bean).
Geographic distribution
India, Africa, Jamaica, Brazil
Biology and transmission
Chlamydospores are black and spherical. Germination by formation of basidia.
Concomitant contamination of seeds.
Detection/indexing methods used at IITA
Seed washing method
- Randomly select a sub sample of 500 seeds (or less if fewer seeds are available) from the seed lot and place in a 250-ml flask.
- Add 100 ml of water and 5 drops of tween 80. Shake vigorously for 30 sec.
- Pour 10 ml of the resulting suspension into a test tube and centrifuge in table-top centrifuge for 5 minutes at 4000 rpm.
- Pour off the supernatant and resuspend the pellet in the bit of remaining water by tapping the tube with your finger. Place of a drop of the suspension on a microscope slide, cover with a cover slip, and inspect the entire slide at X10 and higher under the compound microscope.
- Look for spores, fruiting bodies. Identify
Leaf isolation method/Slide
- Collect infected leaf sample from the field.
- Harvest Entyloma vignaespores from infected leaf or pod samples using a needle on to a clean slide.
- Add a drop of lactophenol or cotton blue
- View under microscope to check for spore identification
Treatment/control
- No effective seed treatment against the pathogen
- Seeds for international distribution are grown in certified PFAs
- Active growth field inspection and certification
- Infected plants rogued
- Seeds not harvested from infected field
Procedures in case of positive test at IITA
- Discard. Not for international distribution in compliance with the importing countries’ phytosanitary regulations
For import or export:
- For important germplasm material to be used for crossing, growing on test in the containment, active growth inspection conducted. Plants with smut symptoms are rogued and incinerated
- Seeds from symptomless plants harvested and retested
|
Leaf infection (photo:IITA) |
Pod infection (photo:IITA) |
Scientific name
Septoria vignae Henn.
Other scientific name
Septoria vignicola Vasant Rao
Importance
High
Significance
Not reported.
Symptoms
Leaf symptoms are very pronounced
Spots coalesce to form dark red circular lesions on both leaf surfaces. Lesions also appear on the pods and stems. Pycnidia are formed in the lesions
 |
 |
|
|
Septoria vignae (photos:IITA) |
||
Hosts
The only major host recorded is Vigna unguiculata (cowpea).
Geographic distribution
India , East Africa, Savannah zones of tropical Africa, Nigeria
Biology and transmission
Fungus produces conidia in pycnidia. Conidia are septate
Seed borne and seed transmitted
Detection/indexing methods used at IITA
- Collect fresh infected samples from the field.
- Surface sterilized with 5% Na0Cl for 2 mins
- Plate infected leaves/ pods/seeds portions on moist filter paper.
- Incubate at 27oC for 24hrs to allow the development of the pycnidiospores.
- Pick the developed pycnidia and plate on PDA infused with lactic acid.
- Incubate the plates at 27oC for 4-6 days.
- Identify under a compound microscope by picking the pycnidia and break it open with a cover slip to identify the spores
Treatment/control
- Seed treatment with mancozeb.
- Seeds for international distribution are grown in certification schemes
- Active growth field inspection.
- Infected plants rogued
Procedures in case of positive test at IITA
- Discard. Not for international distribution in compliance to the importing countries’ phytosanitary regulations
- For import or export: Seeds are treated with mancozeb and retested after 72 hours for presence /absence of pathogen. Infected lines are rejected.
- For important germplasm material to be used for crossing, growing on test in the containment , active growth inspection, plants with Septoria symptoms are rogued and incinerated
Lamptail pod rot of cowpea, Blight of cowpea, Pod rot of cowpea
Scientific name
Choanephora cucurbitarum (Berk. & Ravenel) Thaxt.
Other scientific name
Choanephora americana A. Møller
Importance
High
Significance
The exact data on yield loss are seldom mentionned although in Nigeria. Cowpea losses were estimated at 7-20% by Oladiran (1980).
Symptoms
C. cucurbitarum infects plant tissues that have been damaged either by insects or by physical means. Symptoms vary considerably on different crops
The fungus causes a number of diseases on a range of crops affecting all plant stages: flowering , fruiting , post-harvest, pre-emergence, seedling and vegetative growing and all plant parts; pods, growing points, inflorescence, leaves, seeds, stems and whole plant.
In Nigeria the fungus causes dieback, stem and leaf blight on cowpea.
Visibly field symptoms of Choanephora cucurbitarum on the host tissues have a white hairy appearance resulting from the tall sporangiophores that produce a cluster of brown sporangiola at their tips. On cowpea, symptoms begin as water-soaked lesions at the leaf margins and tips. These lesions became dry and turned olive-green to light brown. Numerous spiny, long sporangiophores developed during dry weather results to total necrosis of the entire plant Turkensteen, 1979; French, 2000.
Symptoms on various plant parts:
Pods: visible mould and whitish spine like mycelium , rot
Growing points: dead heart.
Inflorescence: lesions.
Leaves: lesions; fungal growth; sooty mould; rot; odour.
Seeds: rot.
Stems: external discoloration; canker; dieback; sooty mould.
Whole plant: dieback.
|
Infected pod (photo:IITA) |
Spores |
Hosts
The major hosts attacked by the fungus are: Vigna unguiculata (cowpea), Abelmoschus esculentus (okra), Amaranthus (grain amaranth), Beta vulgaris var. saccharifera (sugarbeet), Brassica oleracea var. botrytis (cauliflower), Cajanus cajan (pigeon pea), Capsicum (peppers), Capsicum annuum (bell pepper), Capsicum frutescens (chilli), Carica papaya (papaw), Citrullus lanatus (watermelon), Cucumis sativus (cucumber), Cucurbita maxima (giant pumpkin), Glycine max (soyabean), Gossypium (cotton), Ipomoea batatas (sweet potato), Manihot esculenta (cassava), Nasturtium officinale (watercress), Phaseolus vulgaris (common bean), Piper nigrum (black pepper), Pisum sativum (pea), Psidium guajava (guava), Psophocarpus tetragonolobus (winged bean), Ricinus communis (castor bean), Sesamum indicum (sesame), Solanum melongena (aubergine), Solanum tuberosum (potato), Sorghum bicolor (sorghum), Spinacia oleracea (spinach), Vigna mungo (black gram), Vigna radiata (mung bean), and Zea mays (maize).
Geographic distribution
The fungus is worldwide in distribution attacking many crops.
Biology and transmission
C. cucurbitarum produces elongate mycelium without septa. The sporangiola, and typical sporangia are sometimes produced but on separate sporangiophores. The zygospores are formed by fusion of two morphologically similar gametes. Zygospores germinate to produce a sporangium containing sporangiospores French, 2000).
C. cucurbitarum has been reported as a pathogen with 48 species belonging to 37 genera within 17 families. It is a weak parasite that grows on predisposed plants whose tissues have been injured mechanically or damaged by insects during feeding (Cuthbert and Fery, 1975) . Mycelium builds up on the affected plant tissues and enzymes are secreted to overcome the resistance of the healthy tissue, which is then invaded (Agrios, 1978). Makambila and Goma, (1993) reported that Sexual spores on Amaranth debris are the main source of inoculum.The fungus produces thick-walled zygospores which can withstand adverse conditions and germinate when temperatures and moisture conditions are favourable for the production of a sporangium containing sporangiospores. These are then disseminated primarily by air currents (Agrios, 1978).
C. cucurbitarum is seedborne on okra in Malaysia (Tai Luang Huan and Musa Bin Jamil, 1975). The seed borne and seed transmitted nature in cowpea has not been established.
Detection/indexing methods used at IITA
- Blotter method.
Procedure
- Randomly select a sub sample of 500 seeds (or less if fewer seeds are available) from the seed lot.
- Surface disinfection of seeds using 10% Sodium hypochlorite for 3 minutes.
- Rinse the seed in sterile distilled water and blot off excess.
- Plate the material on Blotter and incubate at 28oC for 4days.Examine plate under stereo microscope.
- Make microscopic slides of fungal fruiting bodies observed in growth.
- Examine under compound microscope to identify the fungal fruiting bodies and spores isolated from the mycelial growth.
- Subculture on NBY to obtain pure cultures of the pathogen for pathogenicity / preservation.Make microscopic slides of the spores and re examine under the compound microscope for confirmation and purity.
Treatment/control
- Seed treatment with mancozeb
- Seeds for international distribution are grown in certified PFAs
- Active growth field inspection and certification
- Infected plants rogued
- Seeds not harvested from infected field
Procedures in case of positive test at IITA
- Discard. Not for international distribution in compliance to the importing countries’ phytosanitary regulations
- For import or export: Seeds are treated with mancozeb and retested after 72 hours for presence /absence of pathogen. Infected lines are rejected.
- For important germplasm material to be used for crossing, growing on test in the containment , active growth inspection, plants with C. cucurbitarum symptoms are rogued and incinerated
References and further reading
Agrios GN. 1978. Plant pathology. London, UK: Academic Press Inc
CAB International. 2007. Crop Protection Compendium, 2007 Edition. Wallingford, UK.
French ER. 2000. Choanephora blight. In Compendium of Potato Diseases. Second edition. St. Paul, Minnesota, USA: APS Press, in press
Makambila C, Goma JB. 1993. Choanephora cucurbitacearum Curr., a new pathogenic fungus of Amaranthus in Congo. Cahiers Agricultures, 2(3):217-219
Oladiran AO. 1980. Choanephora pod rot of cowpea in southern Nigeria. Tropical Pest Management, 26(4):396-402
Tai Luang Huan, Musa Bin Jamil M. 1975. Seed-borne pathogens in okra fruit rot. MARDI Research Bulletin, 3(2):38-45
Turkensteen LJ. 1979. Choanephora blight of potatoes and other crops grown under tropical conditions in Peru. Netherlands Journal of Plant Pathology, 85(2):85-86
Comments
- No comments found












Leave your comments
Post comment as a guest