stog-wheat
Weeds - wheat
Contributors to this page: CIMMYT, Mexico (Etienne Duveiller, Monica Mezzalama, Eloise Phipps, Thomas Payne, Jesper Norgaard), Independent consultant (Jesse Dubin).
Creeping thistle, Canada thistle
|
Creeping thistle, Canada thistle (photo: ICARDA) |
Scientific name
Cirsium arvense (L.) Scop.
Importance
High.
C. arvense is a noxious weed of the Asteraceae family. It is difficult to eradicate, and is usually introduced accidentally as a contaminant in cereal crop seeds. It is present in Mexico but limited to certain areas. Many countries regulate this plant, or its parts (i.e., seed) as a contaminant.
Significance
C. arvense is a serious invasive species in regions where it has been introduced. It is difficult to eradicate because it produces rhizomes.
Hosts
Cereals and other crops.
Geographic distribution
Native to Europe. Present in the USA, Canada, Mexico, Brazil, South Asia, Brazil, Australia and New Zealand.
Biology
C. arvense is a tall, herbaceous, perennial plant, forming extensive clonal colonies from an underground root system that sends up numerous erect stems each spring, reaching 1-1.2 m tall (occasionally more). The stems often lie partly flat by summer but can stay erect if supported by other vegetation. The leaves are very spiny, lobed, up to 15-20 cm long and 2-3 cm wide (smaller on the upper part of the flower stem). The inflorescence is 10-22 mm in diameter, pink-purple in color, with all the florets of similar form (i.e., with no division into disc and ray florets). The flowers are usually dioecious, but not invariably so, with some plants bearing hermaphrodite flowers. The seeds are 4-5 mm long, with a feathery pappus that assists in wind dispersal.
Most seeds germinate within a year, but buried seed can stay dormant for up to 20 years.
Detection/indexing methods used
- At CIMMYT: Physical inspection of seed
- At ICARDA: Not applicable
Treatment/control
- Killing the roots is the only effective control method in the field. A combination of spring-summer mowing and herbicide application in the fall is extremely effective. There is no seed treatment available
Procedures followed at the centers in case of positive test
- At CIMMYT: Depending on the level of infestation, the seed may be cleaned (i.e., the weed propagules are removed from the wheat seed). If the infestation rate is too high the seed lot is destroyed.
- At ICARDA: Not applicable
References and further reading
Alberta Invasive Plants Council. Invasive alien species factsheet: Canada thistle (Cirsium arvense). [online] Available from URL: http://www.invasiveplants.ab.ca/Downloads/FS-CanadaThistle.pdf. Date accessed 07 April 2010
Blamey M, Grey-Wilson C. 1989. The Illustrated Flora of Britain and Northern Europe. London: Hodder and Stoughton.
United States Department of Agriculture (USDA). PLANTS profile: Cirsium arvense (L.) Scop., Canada thistle. [online] Available from URL: http://www.plants.usda.gov/. Date accessed 07 April 2010
Insects - wheat
Contributors to this page: CIMMYT, Mexico (Etienne Duveiller, Monica Mezzalama, Eloise Phipps, Thomas Payne, Jesper Norgaard), Independent consultant (Jesse Dubin).
Khapra beetle
|
Adult, larva, larval skins of T. granarium |
Scientific name
Trogoderma granarium Everts
Importance
High phytosanitary importance.
Significance
The khapra beetle is considered to be one of the world's most destructive pests of grain products and seeds. It is principally serious under hot dry conditions. Reproduction may be so rapid that larvae are found in large numbers in the surface layers of binned grain. The discovery of T. granarium in a non-infested area usually leads to an immediate quarantine of suspected goods and an expensive eradication and control effort.
Symptoms
Presence in stored grain. The stage most commonly seen during inspection is the larva, and the most usual evidence for infestation is cast larval skins.
Hosts
Grain and stored product hosts.
The beetle is by nature an omnivorous protein scavenger and has been found in many locations that would not be obvious food sources.
Geographic distribution
T. granarium probably originated in the region now including Bangladesh and India. It has since spread to other areas including northern and eastern Africa, southern Europe and the Mediterranean region, and the Middle East.
EPPO distribution map: http://www.eppo.org/QUARANTINE/insects.
Biology and transmission
T. granarium is a member of the Coleoptera family Dermestidae.
The eggs are milky white, turning pale yellowish with age, cylindrical, 0.7 by 0.25 mm, with one end rounded, the other pointed and bearing spine-like projections.
The larvae at hatching are approximately 1.6 to 1.8 mm long, more than half of this length consisting of a tail made up of hairs on the last abdominal segment. Larvae are uniformly yellowish white, except the head and body hairs, which are brown. As the larvae increase in size, their body color changes to a golden or reddish brown, more body hairs develop, and the tail becomes proportionally shorter. Mature larvae are approximately 6 mm long and 1.5 mm wide. Larval development may occur in four weeks, but under conditions of cooler temperatures, crowding, or poor food quality the larvae may enter diapause.
Adults are oval-shaped and brown to black in color, with indistinct lighter brown patterns on the elytra. The adult males are 1.4-2.3 mm long and 0.75-1.1 mm wide; adult females are 2.1-3.4 mm long and 1.7-1.9 mm wide. They appear densely hairy under a microscope. These hairs may trap dust, giving a dirty appearance. Adults are short-lived, persisting only for one to two weeks.
This beetle has never been observed to fly; therefore, its spread is probably dependent on movement of infested goods or in containers where it may be transported while in diapause.
Detection/indexing methods
- At CIMMYT: Physical inspection of seed.
- At ICARDA: Visual inspection
Treatment/control
- Fumigation using methyl bromide is the treatment of choice. Because of the khapra beetle's habit of hiding in cracks and crevices and infesting porous block, the entire storage structure, in addition to its contents, must be fumigated. The future for the continued use of methyl bromide fumigation for khapra beetle is currently uncertain.
Procedures followed at the centers in case of positive test
- At CIMMYT: The seed lot is destroyed (the pest is quarantined in Mexico).
- At ICARDA: Double dose of fumigation with phostoxin
EPPO protocols
EPPO Diagnostic Protocols for Regulated Pests PM 7/13(1): Trogoderma granarium. EPPO Bulletin 32: 241-243. [online] Available from URL: http://www.eppo.org/QUARANTINE/insects/. Date accessed 07 April 2010
References and further reading
Banks HJ. 1977. Distribution and establishment of Trogoderma granarium Everts (Coleoptera: Dermestidae); climatic and other influences. Journal of Stored Product Research 13: 183-202.
Buss LB, Fasulo TR. 2006. UF/IFAS Photo Gallery Stored Product Pests CD-ROM SW 185. Unifersity of Florida IFAS Extension.
EPPO. Data Sheets on Qurantine Pests: Trogoderma granarium. [online] Available from URL:http://www.eppo.org/QUARANTINE/insects/. Date accessed 07 April 2010
French S, Venette RC. 2005. Mini risk assessment: khapra beetle, Trogoderma granarium (Everts) [Coleoptera: Dermestidae]. United States Department of Agriculture. [online] Available from URL: http://www.aphis.usda.gov/ Date accessed 07 April 2010
Lowe S, Browne M, Boudjelas S, DePoorter M. 2000. 100 of the World's Worst Invasive Alien Species: A Selection from the Global Invasive Species Database. Invasive Species Specialist Group, World Conservation Union (IUCN). 12 pp. [online] Available from URL: http://www.issg.org/booklet.pdf. Date accessed 07 April 2010
Szito A. (2006). Trogoderma granarium (insect). Global Invasive Species Database. [online] Available from URL: http://www.issg.org/database/ Date accessed 07 April 2010.
Von Ellenrieder N. 2004. Pest Sheet: Khapra beetle (Trogoderma granarium). Entomology Laboratory, Plant Pest Diagnostics Center, California Department of Food and Agriculture. [online] Available from URL: http://www.cdfa.ca.gov/. Date accessed 07 April 2010
ZipcodeZoo.com. 2009. Trogoderma granarium (khapra beetle). [online] Available from URL: http://zipcodezoo.com/Animals. Date accessed 07 April 2010
Best practices for the safe transfer of wheat germplasm
Contributors to this page: CIMMYT, Mexico (Etienne Duveiller, Monica Mezzalama, Eloise Phipps, Thomas Payne, Jesper Norgaard), Independent consultant (Jesse Dubin).
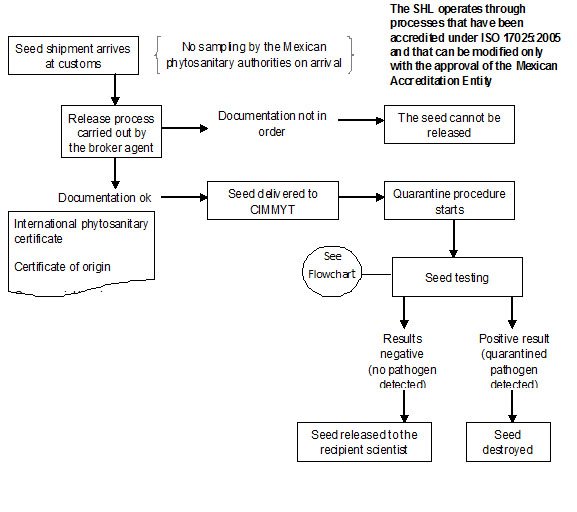
In CIMMYT Mexico, ‘Rules and Regulations for the Safe Movement of wheat and maize germplasm’ have been described by Dr. M. Mezzalama, Head of Seed Health Laboratory (SHL).
Since 1988 the CIMMYT SHL has been officially authorized by DGSV (Direccion General de Sanidad Vegetal) to carry out the quarantine procedures on seed introductions coming into Mexico and CIMMYT, and in April 2007 the SHL obtained accreditation under standard ISO/IEC 17025: 2005, “General requirements for testing and calibration laboratories”, as required by the Mexican government. An official DGSV inspector is assigned exclusively to CIMMYT to assist with thorough and timely seed inspection and importation.
All seed brought into CIMMYT, without exception, must be subjected to quarantine procedures in the Seed Health Laboratory. The seed introduction procedure at CIMMYT Headquarters is summarized health flow chart
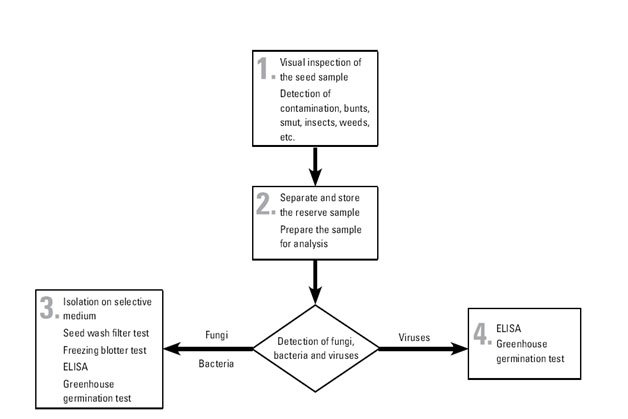
The pathogen detection and identification flowchart is described below:
All wheat and triticale seed, both entering and leaving CIMMYT, must pass through the seed health laboratory. All seed undergoes the same set of testing procedures, although the key target pathogens vary between incoming and outgoing seed depending on relevant quarantine regulations. There is a zero tolerance for Karnal bunt teliospores and very strict procedures are followed for moving seed within Mexico from Karnal bunt-contaminated to Karnal bunt-free areas.
CIMMYT generally uses well-established test procedures that may be found in any standard reference on seed health. The filter wash test is somewhat specialized, and the procedure are given in CIMMYT Seed Health Manual.
The tests used in CIMMYT are as follows:
-
Physical inspection for smut sori, nematode galls, ergot sclerotia, weed seeds, insect damage, etc.
-
Seed wash filter test, which reveals the presence of fungal spores—including bunt teliospores (Tilletia spp.), smut spores (Urocystis and Ustilago spp.), and downy mildew oospores (Peronosclerospora and Sclerophthora spp.)—and of nematode cysts. This test takes around three hours, although large volumes of samples make take longer. Composite samples of outgoing seed may be used (with rechecking of individual lines in the event of a positive result).
-
Freezing blotter test, which reveals the presence of imperfect fungi carried by seed and takes two weeks.
-
Greenhouse germination test, for the expression, and thus detection, of seed-borne pathogens, and to check seed viability. This test takes three weeks. If symptoms appear on seedlings, further testing to identify the causal pathogen is carried out (i.e. ELISA or other tests).
-
ELISA, or enzyme-linked immunosorbent assay, to detect specific bacteria and viruses. This test takes 24 hours.
-
Immunofluoresence test, an immunological assay, taking six hours, for the detection on wheat seed of Xanthomonas translucens pv. undulosa.
Seed healt testing on wheat and triticale at CIMMYT
|
Test
|
Pathogen type(s)
detected
|
Pathogens of importance
in incoming seed
|
Pathogens of quarantine
importance in outgoing seed **
|
|
Seed wash filter test |
Fungi: bunts smuts Nematodes |
Tilletia indica*, Tilletia controversa* Anguina tritici* |
Tilletia indica Tilletia spp Ustilago spp . |
|
Freezing blotter test |
Imperfect fungi |
Alternaria triticina* |
Fusarium spp. Helminthosporium spp. Septoria spp. |
|
Greenhouse germination test |
Bacteria Viruses |
Pseudomonas syringae pv. atrofaciens* Xanthomonas translucens pv. undulosa Barley stripe mosaic virus
Wheat streak mosaic virus |
P. syringae |
|
ELISA |
Viruses |
Barley stripe mosaic virus Wheat streak mosaic virus |
Barley stripe mosaic virus Wheat streak mosaic virus |
|
Immunofluorescence
|
|
Xanthomonas translucens pv. undulosa |
|
* Quarantined under Norma Oficial Mexicana NOM-017-FITO-1995.
** According to information reported on importing countries requirements
References and further reading
Mezzalama M. 2012. Seed Health: Fostering the Safe Distribution of Maize and Wheat Seed: General guidelines. Third edition. Mexico, D.F.: CIMMYT. Available here (0,5 MB)
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. 84 pp
Viruses - wheat
Contributors to this page: CIMMYT, Mexico (Etienne Duveiller, Monica Mezzalama, Eloise Phipps, Thomas Payne, Jesper Norgaard), Independent consultant (Jesse Dubin).
|
Contents: |
Barley Stripe Mosaic Virus (BSMV)
Importance
Virus of Gramineae efficiently transmitted through seed. High phytosanitary importance.
Significance
Rare in wheat, causing negligible economic losses. Yield losses are proportional to the level of infection in the seed lot.
Symptoms
Symptoms may vary from very mild stripe mosaic to lethal necrosis depending on the host genotype and environmental conditions (temperatures between 24 and 30°C favor disease development). Symptoms may be confused with barley stripe disease (Pyrenophora graminea).
Infected plants may also exhibit dwarfing, resetting, and excessive tillering.
|
Barley stripe mosaic (photo: CIMMYT) |
Hosts
Natural hosts known are barley, wheat and wild oat.
Geographic distribution
Cosmopolitan
Biology and transmission
No known vectors transmit BSMV. The virus is transmitted through seed, pollen, or direct leaf contact. The main route of natural spread of the virus in the field seems to be by plant-to-plant contact. The seed transmission rate in barley can reach up to 100%.
Mechanical inoculation of the plant species Chenopodium amaranticolor or C. quinoa with the virus produces local lesions, which are useful for detection.
Detection/indexing method
- At CIMMYT: Detection with ELISA using Agdia protocol (http://www.agdia.com).
- At ICARDA: Enzyme-linked immunosorbent assay (ELISA), (TBIA) Tissue Blot ImmunoAssay test
Treatment/control
- No direct treatment available.
Procedures followed at the centers in case of positive test
- At CIMMYT: The seed lot is destroyed.
- At ICARDA: The seed lot is destroyed
EPPO protocols
EPPO Phytosanitary Procedure PM 3/34(1): Barley stripe mosaic hordeivirus. Inspection and test methods for barley seeds. [online] Available from URL: at: http://www.eppo.org/STANDARDS/standards.htm Date accessed 12 March 2010
References and further reading
Albrechtsen SE. 2006. Testing Methods for Seed-Transmitted Viruses: Principles and Protocols. Wallingford, UK: CAB International. 268 pp.
Albrechtsen SE. 1993. Viral diseases. In S.B. Mathur and B.M. Cunfer (eds.), Seed-borne Diseases and Seed Health Testing of Wheat. Copenhagen: Danish Government Institute of Seed Pathology for Developing Countries. pp. 147-151.
Diekmann M, Putter CAJ. (eds.). 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14: Small Grain Temperate Cereals. Rome: Food and Agriculture Organization of the United Nations (FAO) and International Plant Genetic Resources Institute (IPGRI). pp. 12-13.
Lapierre H, Signoret PA. (eds.). 2004. Viruses and Virus Diseases of Poaceae (Gramineae). Paris: INRA. pp. 456-457.
Lister RM, Carroll TW, Zaske SK. 1981. Sensitive serologic detection of barley stripe mosaic virus in barley seed. Plant Disease 65: 809-814.
Wiese MV. 1987. Compendium of wheat diseases. 2nd edn. St Paul, MN: APS Press. pp. 69-70.
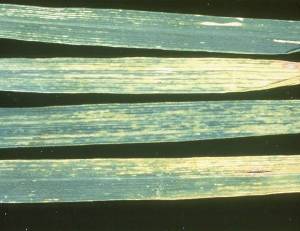
Wheat Streak Mosaic Virus (WSMV)
|
Wheat Streak Mosaic Virus symptoms(WSMV) (photo top: ICARDA, photo middle and bottom: CIMMYT) |
Importance
High phytosanitary importance. WSMV is an important and widely distributed wheat disease with seed transmission. Present in Mexico.
Significance
High economic importance. Losses vary from insignificant to complete.
Symptoms
Infected plants are stunted and develop a rosette appearance. Leaves are mottled and streaked. Leaf streaks are green to yellow, parallel and discontinuous.
Symptoms are not usually apparent in autumn-sown plants or in early spring, but become noticeable when temperatures rise above 10°C.
Hosts
Wheat, maize, millet and various grasses.
Geographic distribution
The USA, Mexico, Europe, parts of Russia, and recently Australia.l
Biology and transmission
Particles of WSMV, a potyvirus, are difficult to purify and quantify. It is terminally inactivated at 54°C.
It is transmitted by the wheat curl mite (Aceria tulipae). Seed transmission was not thought to occur until recently, when the virus was unequivocally shown to be transmitted to a small but consistent proportion of seedlings grown from WSMV-infected wheat plants (Jones et al. 2005).
The disease is severe in areas with a year-round continuum of host plants.
Detection/indexing method
- At CIMMYT: Detection with ELISA using Agdia protocol (http://www.agdia.com).
- At ICARDA: Not applicable
Treatment/control
- No direct treatment available.
- Wheat streak mosaic is controlled by cultural practices that minimize the sources of WSMV and mites at the emergence of new wheat crops, such as the destruction of volunteers.
Procedures followed at the centers in case of positive test
- At CIMMYT: The seed lot is destroyed.
- At ICARDA: Not applicable.
References and further reading
Albrechtsen SE. 2006. Testing Methods for Seed-Transmitted Viruses: Principles and Protocols. Wallingford, UK: CAB International. 268 pp.
CIMMYT. Wheat Doctor information sheet: Wheat streak mosaic virus (WSMV). [online] Available from URL: http://wheatdoctor.cimmyt.org. Date accessed 06 April 2010
Dwyer GI, Gibbs MJ, Gibbs AJ, Jones RAC. 2007. Wheat streak mosaic virus in Australia: Relationship to isolates from the Pacific Northwest of the USA and its dispersion via seed transmission. Plant Disease 91: 164-170.
French R, Stenger DC. 2003. Evolution of wheat streak mosaic virus: Dynamics of population growth within plants may explain limited variation. Annu. Rev. Phytopathol. 41: 199-214.
Jones RAC, Coutts BA, Mackie AE, Dwyer GI. 2005. Seed transmission of wheat streak mosaic virus shown unequivocally in wheat. Plant Disease 89: 1048-1050.
Lapierre H, Signoret PA. (eds.). 2004. Viruses and Virus Diseases of Poaceae (Gramineae). Paris: INRA. pp. 602-604.
Sanchez-Sanchez H, Henry M, Cardenas-Soariano E, Alvizo-Villasana HF. 2001. Identification of wheat streak mosaic virus and its vector Aceria toschella. Plant Disease 85: 13-17.
Wiese MV. 1987. Compendium of wheat diseases. 2nd edn. St Paul, MN: APS Press. pp. 80-81.
Nematodes - wheat
Contributors to this page: CIMMYT, Mexico (Etienne Duveiller, Monica Mezzalama, Eloise Phipps, Thomas Payne, Jesper Norgaard), Independent consultant (Jesse Dubin).
Seed gall nematode, wheat nematode
Causes ear cockle and yellow slime or tundu
|
Seed Gall Nematode (also known as Wheat Nematode or Ear Cockle) As diseased plants
Seed Gall Nematode (also known as Wheat |
Scientific name
Anguina tritici (Steinbuch) Chitwood
Importance
The pest should not be a problem where good seed production systems are in place. It is extinct or rare in most parts of Europe, Australasia, Russia and North America.
Some countries consider Anguina tritici an important quarantine/quality concern and impose a nil tolerance for galls in imported grain. Trade implications are the major impact of this nematode.
Significance
This nematode is rarely of economic importance.
Symptoms
Distorted leaves and stems are evident prior to heading. As diseased plants approach maturity, galls are formed in the florets, replacing the kernels. Seed galls in cereals are known as ear cockle. The galls are similar in shape to the seed they replace but are dark brown in color. Large numbers of motile larvae are present within the galls and become active after the galls have been moistened.
Although potentially an important pest in its own right, Anguina tritici also acts as a vector for Rathayibacter tritici, which causes yellow slime rot or tundu.
Hosts
The seed gall nematode parasitizes wheat, triticale, rye, and related grasses; it primarily affects wheat.
Geographic distribution
Ear cockle was in the past reported in all the major wheat-growing areas. However, Anguina tritici has now become rare or locally extinct in many countries. It is still found in the Near and Middle East, the Asian Subcontinent, China, and parts of Africa.
Biology and transmission
The female is about 3.8 mm long and spirally coiled. Although females exhibit some movement, they are effectively immobile. The stylet is relatively short at 8 to 10 μm long. Males are not as robust as the females, curved rather than coiled and only about 2.4 mm long.
At maturity, the galls induced by the nematode contain large numbers of second stage juveniles (J2s, c. 800 μm long), which are the survival, dispersal and invasive stage of Anguina tritici. Galls already in the soil or sown with contaminated seeds are the sources of infestation. In the field, galls become moist and soft, facilitating release of second stage juveniles. Juveniles move progressively to the growing tip until they penetrate the floral primordia, where they develop to maturity. Females lay thousands of eggs in the developing seed gall; these hatch and when the juveniles reach the J2 stage they enter anhydrobiosis. There is one generation per year.
Detection/indexing methods used
- At CIMMYT: Physical inspection of seed.
- At ICARDA: Visual inspection, nematode soaking test
Treatment/control
- Seed cleaning and crop rotation together can achieve complete control to the point of localised extinction. Modern harvesting, seed cleaning and testing practices are more than adequate to ensure that seed is free of the nematode. The most effective control is by mechanical seed cleaning. Galls may be also removed by submersion of the seed in 20% brine solution (galls float to the surface), followed by thorough washing in water. Hot water treatment at 54°C for 10 min is also reported to be effective in killing the nematodes.
- Modern harvesting, seed cleaning and testing practices are more than adequate to ensure seed free of the nematode. For small seed lots, where this technology is not available, brine (10%) flotation is more efficient than hand sieving.
- In addition to clean seed, carryover from one crop to the next in the same or adjacent fields needs to be avoided. Despite having a remarkable longevity when stored dry, only a small proportion of juveniles will survive to a second season in the field, hence rotation with a non-host crop and control of volunteer wheat will provide effective field control.
Procedures followed at the centers in case of positive test
- At CIMMYT: The seed lot is destroyed (the nematode is quarantined in Mexico).
- At ICARDA: Infected kernels are eliminated
References and further reading
CIMMYT. Wheat Doctor information sheet: Seed gall nematode. [online] Available fromURL: http://wheatdoctor.cimmyt.org/. Date accessed 07 April 2010
Diekmann M, Putter CAJ. (eds.). 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14: Small Grain Temperate Cereals. Rome: Food and Agriculture Organization of the United Nations (FAO) and International Plant Genetic Resources Institute (IPGRI). pp. 53-54.
Paruthi IS, Bhatti DS. 1985. Estimation of loss in yield and incidence of Anguina triticina on wheat in Haryana (India). International Nematology Network Newsletter 2: 13-16.
Prescott JM, Burnett PA, Saari EE, Ransom J, Bowman J, de Milliano W, Singh RP, Bekele G. 1986. Wheat Diseases and Pests: A Guide for Field Identification. Mexico, D.F.: CIMMYT. pp. 98-99. [online] Available from URL: http://www.cimmyt.org/. Date accessed 07 April 2010
Riley IT, Reardon TB. 1995. Isolation and characterization of Clavibacter tritici associated with Anguina tritici in wheat from Western Australia. Plant Pathology 44: 805-810.
Southey JF, Topham PB, Brown DJF. 1990. Taxonomy of some species of Anguina Scopoli, 1777 (sensu Brzeski, 1981) forming galls on Gramineae: value of diagnostic characters and present status of nominal species. Revue de Nematologie 13: 127-142.
Swarup G, Sethi CL, Gotke N. 1993. Ear cockle (nematode gall) and yellow slime. In: Mathur SB, Cunfer BM. (eds.), Seed-borne Diseases and Seed Health Testing of Wheat. Copenhagen: Danish Government Institute of Seed Pathology for Developing Countries. pp. 153-161.
Wiese MV. 1987. Compendium of wheat diseases. 2nd edn. St Paul, MN: APS Press. pp. 63-64.







.jpg)