stog-maize
Insects - maize
Contributors to this section: CIMMYT, Mexico (Etienne Duveiller, Monica Mezzalama, Eloise Phipps, Thomas Payne, Jesper Norgaard), Independent consultant (Jesse Dubin).
Khapra beetle
Importance
High economic and phytosanitary importance.
Significance
Trogoderma granarium is a general storage pest. It occurs mainly on cereals and cereal products, oilseeds (especially groundnuts and oilcakes), pulses and pulse products, and compound animal feeding stuffs. It is principally serious under hot dry conditions; complete destruction of grain and pulses may take place in a short time. In humid climates, it is not competitive with other storage pests with faster rates of increase.
Symptoms
Presence in stored grain. The stage most commonly seen during inspection is the larva, and the most usual evidence for infestation is cast larval skins.
 Trogoderma granarium (photo: EPPO) |
Hosts
Grain and stored product hosts.
The beetle is by nature an omnivorous protein scavenger and has been found in many locations that would not be obvious food sources.
Geographic distribution
Trogoderma granarium is established within an area roughly limited by the 35° parallel to the north, the Equator to the south, West Africa to the west and Myanmar to the east; i.e. the warm dry regions along the Suez route from the Indian subcontinent to Europe. T. granarium has been introduced into other areas with similar climatic conditions, especially the alternative route between India and Europe around Africa. Initially, these introductions caused severe damage but outbreaks have been local and most have been eradicated. T. granarium has also established in some areas with unfavorable climates, in protected storage environments only, for example in Western Europe and Japan.
EPPO distribution map: http://www.eppo.org/QUARANTINE/insects/Trogoderma_granarium/TROGGA_map.htm
Biology and transmission
The natural spread of this pest is limited. International spread is mainly by larvae in commodities, empty sacks, and in the structure of ships and dry cargo containers. Second-hand bags are also a means of spread.
Detection/indexing methods used at CIMMYT
- Physical inspection of seed.
Treatment/control
- Fumigation
Procedures followed in case of positive test used at CIMMYT
- The seed lot is destroyed (the insect is quarantined in Mexico).
EPPO protocols
EPPO Diagnostic Protocols for Regulated Pests PM 7/13(1): Trogoderma granarium. EPPO Bulletin 32: 241-243. [online] Available from URL: http://www.eppo.org/QUARANTINE/insects/Trogoderma_granarium/TROGGA_protocol.pdf. Date accessed 10 April 2010
References and futher reading
CAB International. 2007. Datasheet: Trogoderma granarium. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International.
EPPO. Data Sheets on Quarantine Pests: Trogoderma granarium. [online] Available from URL: http://www.eppo.org/QUARANTINE/insects/Trogoderma_granarium/TROGGA_ds.pdf. Date accessed 10 April 2010
Larger grain borer
Importance
High economic and phytosanitary importance.
Significance
Infestations in maize may start on the mature crop in the field, i.e. when moisture content is at or below 18%. Weight losses of up to 40% have been recorded in Nicaragua from maize cobs stored on the farm for 6 months. In Tanzania, up to 34% losses have been observed after 3 months storage on the farm, with an average loss of 8.7%.
Symptoms
These insects infest both stored grain and maturing maize ears in the field. In a very short time, the adults produce large quantities of floury dust as they bore into and feed on the grains. Damaged grains can readily be identified since they are usually covered by a film of this dust.
The adult beetles are 3 to 5mm long, cylindrical in shape and reddish brown to dark brown. Their heads are turned down and appear to be covered by a hood. They are able to fly.
 Prostephanus truncates |
Hosts
Major hosts: maize, cassava (Manihot esculenta), dried stored products, wood.
Minor hosts: Dioscorea (yam), Sorghum bicolor (sorghum), triticale, wheat.
Geographic distribution
P. truncatus is indigenous in Central America, tropical South America, and the extreme south of the USA as a major, but localized, pest of farm-stored maize. It was introduced into Tanzania, probably in the late 1970s, and has become a serious pest of stored maize and dried cassava in that part of East Africa; it has since spread into Kenya, Burundi, Rwanda, Malawi, Zambia, Mozambique, Namibia and South Africa, and is almost certainly present but unreported in several other countries in the region. It was first found in West Africa in Togo in 1984 and it has since spread to Benin, Nigeria, Ghana, Niger and Burkina Faso. A separate outbreak occurred in Guinea in Conakry.>/p>
Biology and transmission
P. truncatus may be attracted to maize grain and dried cassava over short distances. However, field studies in both Mexico and Togo suggest that there is no long-range attraction of adult P. truncatus to maize grain or cobs, or dried cassava; this is not surprising because wood is the major host of this beetle.
Adults frequently initiate their attack on stored maize cobs with intact sheaths by boring into the base of the maize cob core, eventually gaining access to the grain via the apex of the cob by crawling between the sheathing leaves. Adults bore into the maize grains, making neat round holes, and as they tunnel from grain to grain they generate large quantities of maize dust. Adult females lay eggs in chambers bored at right angles to the main tunnels. Egg-laying on stabilized grain, like that on the maize cob, is more productive than on loose-shelled grain as the oviposition period is longer, equal in length to the life of the female, and the eggs are laid at a greater rate.
Larvae hatch from the eggs after about three days at 27°C and seem to thrive on the dust produced by boring adults. For example, large numbers of larvae develop and pupate in dust at the base of dense laboratory cultures. Larvae may also crawl into and feed on slightly damaged kernels. Pupation takes place inside the kernels, and emerging adults then cut their way out of the kernels.
The success of this pest may be partly due to its ability to develop in grain in low moisture conditions, under which many other storage pests are unable to multiply.. For example, Sitophilus oryzae, a species occurring in a very similar ecological niche, needs a grain moisture content of at least 10.5% for development. Thus, in dry conditions, P. truncatus probably benefits from the absence of any significant competition from other storage pests.
Detection/indexing methods used at CIMMYT
- Physical inspection of seed.
Treatment/control
- Fumigation; resistance
Procedures following in case of positive test used at CIMMYT
- The seed lot is destroyed.
References and further reading
CAB International. 2007. Datasheet: Prostephanus truncatus. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International.
CIMMYT. Maize Doctor information sheet: Grain borers. [online] Available from URL:http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=221. Extended information sheet: Grain borers. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=329. Date accessed 10 April 2010
Viruses - maize
Contributors to this section: CIMMYT, Mexico (Etienne Duveiller, Monica Mezzalama, Eloise Phipps), Independent consultant (Jesse Dubin).
|
Contents: |
Pathogen name
Maize dwarf mosaic virus (MDMV)
Importance
High economic importance, moderate phytosanitary importance.
Significance
Maize yield can be severely affected by MDMV. Yield losses of maize in the field can be as high as 40%. Moreover, in maize inbred lines or sweetcorn, especially after later sowing, yield can be reduced by 75% or more. Several crop loss studies in the USA, particularly with artificial inoculation, have reported yield reductions of 70 to 90%. In combination with maize chlorotic mottle virus, MDMV may also cause a severe reaction known as maize lethal necrosis.
Symptoms
Symptoms vary widely depending on host genotype and time of infection. Generally, infected plants develop a distinct mosaic—irregularities in the distribution of normal green color—typically appearing at the bases of the youngest leaves. There may also be narrow chlorotic streaks extending parallel to the veins. Later, the youngest leaves show a general chlorosis, and streaks are larger and more abundant. Reddish-violet discoloration and necrosis may also occur, particularly in sweetcorn and maize inbred lines. Any leaves that are already mature at the time of infection are not affected.
Infected plants are characterized by stunting (of variable severity) and shortening of upper internodes. Sterility is a common symptom of MDMV, often reaching 25% or more. In maize inbred lines, especially after an early infection, sterility can be much higher, causing complete or very high yield loss. Ear development can also be arrested, leading to incomplete grain filling. Plants infected early may produce nubbins or be totally barren. In severe cases the virus can cause premature plant death.
 Maize dwarf mosaic (photo: CIMMYT) |
Hosts
Major hosts: maize, Johnson grass (Sorghum halepense).
Minor hosts: oats (Avena sativa), millet (Panicum miliaceum), sugarcane (Saccharum officinarum).
Wild hosts: prairiegrass (Bromus catharticus), purpletop chloris (Chloris barbata), sour paspalum (Paspalum conjugatum), pearl millet (Pennisetum glaucum), Sudan grass (Sorghum sudanense), buffalo grass (Stenotaphrum secundatum), eastern gamagrass (Tripsacum dactyloides), marmalade grass (Urochloa plantaginea).
Geographic distribution
Worldwide.
Biology and transmission
Numerous aphid species (both adults and nymphs) transmit MDMV in a non-persistent manner. The virus is acquired by the aphid within ten seconds to two minutes of feeding and a latent period is not required. The virus overwinters in alternate hosts. The virus can be sap-transmitted under laboratory conditions. It is also transmitted by seed at low rates.
Detection/indexing methods used at CIMMYT
- Germination test on sterile soil.
- Detection with ELISA using AGDIA protocol (http://www.agdia.com).
Treatment/control
- Resistance, cultural practices, insecticides
Procedures followed in case of positive test used at CIMMYT
The seed lot is destroyed.
References and further reading
CAB International. 2007. Datasheet: Maize dwarf mosaic virus. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International.
CIMMYT. Maize Doctor information sheet: Maize dwarf mosaic virus. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=209. Extended information sheet: Maize dwarf mosaic virus. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=238. Date accessed 10 April 2010
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 38-40.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 94-95.
Pathogen name
Sugarcane mosaic virus (SCMV)
Importance
Moderate economic importance, low phytosanitary importance.
Significance
Yield losses due can be extensive, and in China the virus has been reported to seriously affect maize production. In Germany, SCMV was found to be more prevalent than MDMV, and to have a similar effect on growth and yield of maize. Early infection reduced plant height by 25%, total weight by 38%, and ear weight by 27%. In East Africa, inoculation with SCMV at the seedling stage caused yield losses of 18-46% in ten susceptible maize hybrids. Inoculation with the virus in Australia caused similar losses of up to 50%.
Symptoms
Symptoms vary widely depending on host genotype and time of infection. Generally, infected plants develop a distinct mosaic—irregularities in the distribution of normal green color—typically appearing at the bases of the youngest leaves. There may also be narrow chlorotic streaks extending parallel to the veins. Later, the youngest leaves show a general chlorosis, and streaks are larger and more abundant. Reddish-violet discoloration and necrosis may also occur, particularly in sweetcorn and maize inbred lines. Any leaves that are already mature at the time of infection are not affected.
Infected plants are characterized by stunting (of variable severity) and shortening of upper internodes. Sterility is a common symptom of MDMV, often reaching 25% or more. In maize inbred lines, especially after an early infection, sterility can be much higher, causing complete or very high yield loss. Ear development can also be arrested, leading to incomplete grain filling. Plants infected early may produce nubbins or be totally barren. In severe cases the virus can cause premature plant death.
 Sugarcane mosaic of maize (photo: CIMMYT) |
Hosts
Major hosts: maize, sugarcane (Saccharum officinarum), Johnson grass (Sorghum halepense).
Wild hosts: numerous grasses (Poaceae).
Geographic distribution
Worldwide.
Biology and transmission
Numerous aphid species (both adults and nymphs) transmit SCMV in a non persistent manner, with no latent period required. The virus overwinters in alternate hosts. It is also transmitted by seed at low rates.
Detection/indexing methods used at CIMMYT
- Germination test on sterile soil
- Detection with ELISA using AGDIA protocol (http://www.agdia.com).
Treatment/control
- Insecticides for aphid control
Procedures followed in case of positive test used at CIMMYT
- The seed lot is destroyed.
References and further reading
CAB International. 2007. Datasheet: Sugarcane mosaic virus. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International.
CIMMYT. Maize Doctor information sheet: MDMV/SCMV. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=209. Extended information sheet: MDMV/SCMV. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=238. Date accessed 10 April 2010
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 38-40.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 94-95.
Pathogen name
Maize chlorotic mottle virus (MCMV)
Importance
High economic importance, low phytosanitary importance.
Significance
The disease was first reported in Peru in 1973, where it can causes losses of 10-15% in some cultivars. Inoculation reduced yields by 50%. The combination of MCMV with maize dwarf mosaic virus or wheat streak mosaic virus may also cause a severe reaction known as maize lethal necrosis (MLN).
Symptoms
Fine chlorotic spots, appearing first on the youngest leaves, which coalesce and develop into broad chlorotic stripes along the veins. These chlorotic stripes contrast with dark green tissue when observed against the light. Affected leaves later die. Infected plants are stunted because of shortened internodes, and produce fewer and smaller ears. In most cases the tassel is malformed.
 Maize chlorotic mottle virus (MCMV)(photo: CIMMYT) |
Hosts
Maize, teosinte.
Geographic distribution
USA, Mexico, Argentina, Brazil, Peru.
Biology and transmission
The virus is transmitted mainly by several species of chysromelid leaf beetle, such as the corn flea beetle (Chaetocmena pulicaria), the cereal leaf beetle (Oulema melanopa), and corn rootworms (Diabrotica spp). The virus overwinters on crop residues; beetle larvae that feed on the residues in the absence of fresh material later transmit the virus to the new crop. Reports indicate that it is also transmitted at very low rates via infected seed.
Detection/indexing methods used at CIMMYT
- Germination test on sterile soil.
- Detection with ELISA using AGDIA protocol (http://www.agdia.com).
Treatment/control
- Insect control and cultural practices
Procedures followed in case of positive test used at CIMMYT
- The seed lot is destroyed.
References and further reading
CAB International. 2007. Datasheet: Maize chlorotic mottle virus. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International.
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 124-125.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 92-93.
Nematodes - maize
Contributors to this section: CIMMYT, Mexico (Etienne Duveiller, Monica Mezzalama, Eloise Phipps, Thomas Payne, Jesper Norgaard), Independent consultant (Jesse Dubin).
Maize cyst nematode, corn cyst nematode
Scientific Name
Heterodera zeae
Importance
High economic importance, moderate phytosanitary importance.
Significance
H. zeae is widely distributed in India, Pakistan and Egypt and is presently growing in importance since its detection in the USA, where maize is cultivated over large areas. In India it is considered to be one of the four most important cyst nematodes in that country and is an economic pest of maize. Although accurate estimates of economic crop loss are not available, maize yield has been reported to decline with infestation even when the plants have been exposed to good cultural and fertilization practices, indicating that the decline in yield is due mainly to infestation by H. zeae. Losses appear to be greater in coarse-textured than in fine-textured soil, and greater during hot, dry seasons than in cool, wet seasons.
Symptoms
As with other cyst nematodes, the presence of cysts on the root surface is the most important characteristic used in the identification of H. zeae. Therefore, the most important stage for identification is after the formation of cysts. Above-ground symptoms are very similar to those brought about by other plant parasitic nematodes and include patchy areas with unthrifty, stunted plants having chlorotic and narrowed leaves.
 Corn cyst nematode damages (Jonathan D. Eisenback, Virginia |
Hosts
Major hosts: maize.
Minor hosts: oats (Avena sativa), barley (Hordeum vulgare), rice (Oryza sativa), foxtail millet (Setaria italica), sorghum (Sorghum bicolor), Sudan grass (Sorghum sudanense), wheat, teosinte.
Wild hosts: grasses (Poaceae).
Geographic distribution
India, Pakistan, Egypt, and the USA.
Biology and transmission
H. zeae is reported to have both endoparasitic and semi-endoparasitic habits. The adult females are sedentary and remain within or attached semi-endoparasitically to the roots.
Temperature and soil type play important roles in the biology of H. zeae. As is the case with other Heterodera species, the root diffusate leaching out from the growing roots of host plants also significantly influences the hatching of the eggs. The optimal temperature for the emergence of juveniles from cysts is thought to be between 25 and 33°C. The life cycle is very short and H. zeae is able to complete about 6-7 generations during one cropping season.
Spread of H. zeae by itself is limited. Movement results mainly from flooding, drainage or other forms of transport, soil washings, farm machinery, livestock, seed, grain, tools or people. Every effort should therefore be taken to ensure the prevention of transport of infested material to hitherto uninfested areas and to maintain high standards of sanitation.
Detection/indexing methods used at CIMMYT
- Physical inspection of seed.
Treatment/control
- No seed treatment
Procedures followed in case of positive test used at CIMMYT
- The seed lot is destroyed (the pathogen is quarantined in Mexico).
References and further reading
CAB International. 2007. Datasheet: Heterodera zeae. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International.
Best practices for the safe transfer of maize germplasm
Contributors to this page: CIMMYT, Mexico (Etienne Duveiller, Monica Mezzalama, Eloise Phipps, Thomas Payne, Jesper Norgaard), Independent consultant (Jesse Dubin).
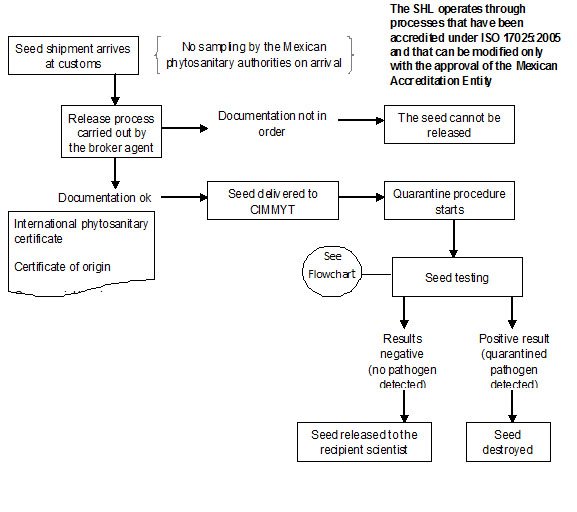
In CIMMYT Mexico, ‘Rules and Regulations for the Safe Movement of wheat and maize germplasm’ have been described by Dr. M. Mezzalama, Head of Seed Health Laboratory (SHL).
Since 1988 the CIMMYT SHL has been officially authorized by DGSV (Direccion General de Sanidad Vegetal) to carry out the quarantine procedures on seed introductions coming into Mexico and CIMMYT, and in April 2007 the SHL obtained accreditation under standard ISO/IEC 17025: 2005, “General requirements for testing and calibration laboratories”, as required by the Mexican government. An official DGSV inspector is assigned exclusively to CIMMYT to assist with thorough and timely seed inspection and importation.
All seed brought into CIMMYT, without exception, must be subjected to quarantine procedures in the Seed Health Laboratory.
The seed introduction procedure at CIMMYT Headquarters is summarized health flow chart
The pathogen detection and identification flowchart is described below:
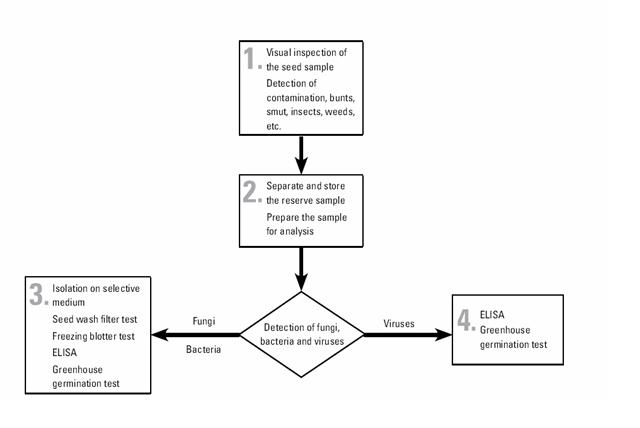
All maize seed, both entering and leaving CIMMYT, must pass through the seed health laboratory. All seed undergoes the same set of testing procedures, although the key target pathogens vary between incoming and outgoing seed depending on relevant quarantine regulations.
CIMMYT generally uses well-established test procedures that may be found in any standard reference on seed health. The filter wash test is somewhat specialized, and the procedure are given in CIMMYT Seed Health Manual.
The tests used in CIMMYT are as follows:
- physical inspection for smut sori, nematode galls, ergot sclerotia, weed seeds, insect damage, etc.
- Seed wash filter test, which reveals the presence of fungal spores—including bunt teliospores (Tilletia spp.), smut spores (Urocystis and Ustilago spp.), and downy mildew oospores (Peronosclerospora and Sclerophthora spp.)—and of nematode cysts. This test takes around three hours, although large volumes of samples make take longer. Composite samples of outgoing seed may be used (with rechecking of individual lines in the event of a positive result).
- Freezing blotter test, which reveals the presence of imperfect fungi carried by seed and takes two weeks.
- Greenhouse germination test, for the expression, and thus detection, of seed-borne pathogens, and to check seed viability. This test takes three weeks. If symptoms appear on seedlings, further testing to identify the causal pathogen is carried out (i.e. ELISA or other tests).
- ELISA, or enzyme-linked immunosorbent assay, to detect specific bacteria and viruses. This test takes 24 hours.
- Downy mildew detection test. This test, with microscopic examination, detects Peronosclerospora and Sclerophthora spp on maize. It takes 24 hours.
Seed health testing on maize
|
Test
|
Pathogen type(s) detected
|
Pathogens of importance in incoming seed
|
Pathogens of quarantine importance in outgoing seed **
|
|
Seed wash filter test |
Fungi ( smuts) Nematodes |
Heterodera zeae* |
Ustilago maydis
|
|
Freezing blotter test
|
Imperfect fungi
|
Acremonium maydis*
|
Cochliobolus spp. Dilpodia spp. Fusarium spp. Lasiodiplodia theobromae |
|
Greenhouse germination test |
Bacteria
Viruses
|
Burkholderia andropogonis* Clavibacter michiganensis subsp. nebraskensis* Pantoea stewartii* Wheat streak mosaic virus*
|
Acidovorax aveanae subsp.avenae C. michiganensis subsp. nebraskensis P. stewartii Maize dwarf mosaic virus Maize chlorotic dwarf virus Sugarcane mosaic virus |
|
ELISA |
Bacteria
Viruses
|
Pantoea stewartii*
Wheat streak mosaic virus*
|
P. stewartii
Maize dwarf mosaic virus Maize chlorotic dwarf virus Sugarcane mosaic virus |
|
Downy mildew detection test |
|
Peronosclerospora maydis* P. philippinensis* P. sacchari* P. sorghi Sclerophthora rayssiae var. zeae* |
P. sorghi
|
* Quarantined under Norma Oficial Mexicana NOM-018-FITO-1995.
** According to information reported on importing countries requirements
References and further reading
Mezzalama M. 2012. Seed Health: Fostering the Safe Distribution of Maize and Wheat Seed: General guidelines. Third edition. Mexico, D.F.: CIMMYT. Available here (0,5 MB)
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. 84 pp
Fungi - maize
Contributors to this section: CIMMYT, Mexico (Etienne Duveiller, Monica Mezzalama, Eloise Phipps), Independent consultant (Jesse Dubin).
Late wilt, black bundle disease of maize
Pathogen name
Acremonium maydis
Other scientific names
Cephalosporium maydis
Importance
Moderate phytosanitary importance, high potential economic importance.
Significance
Widespread incidence and severity in Egypt, with 100% infection reported in some fields. Has also been found in India. Does not occur elsewhere, but considered potentially an important pathogen.
Symptoms
Late-season wilting: no symptoms until plants reach tasseling stage, when wilting generally begins from the top leaves. Leaves turn dull green and dry. Stalks become dry, shrunken and hollow, with discoloration of the vascular bundles starting in the underground portion of the roots. Diseased plants produce only nubbins or ears with underdeveloped, shrunken kernels.
 Black bundle disease (photo: CIMMYT) |
Hosts
Maize.
Geographic distribution
Egypt, India.
Biology and transmission
The pathogen is soilborne, with sclerotia surviving on crop residues. It is seedborne, but not seed-transmitted.
Detection/indexing methods used at CIMMYT
- Freezing blotter test.
Treatment/control
- Resistance, rotation, soil fertility.
Procedures followed in case of positive test used at CIMMYT
- The seed lot is destroyed (the pathogen is quarantined in Mexico).
References and further reading
CAB International. 2007. Datasheet: Acremonium maydis. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 66-67.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 52-53.
Maize doctor: http://maizedoctor.cimmyt.org/
Pathogen name
Claviceps gigantea (anamorph Sphacelia sp.)
Importance
Moderate to low economic and phytosanitary importance.
Significance
The disease is confined to high humid valleys of central Mexico, where it is endemic. The disease is rarely found but when it occurs infection rates are high; infection on 46-53% of ears has been detected. The presence of one sclerotium can reduce seed germination of 50%. The pathogen also produces toxic alkaloids.
Symptoms
Infected kernels grow into large fungal structures, several times the length of normal seeds, known as sclerotia. These are initially pale-colored, soft, slimy/sticky and hollow. Towards harvest they become hard and horny, white to brown, and often resemble a horse’s tooth. Seeds adjacent to those replaced by sclerotia shrivel and become coffee-colored, and have reduced germination.
 Ergot (photo: CIMMYT) |
Hosts
Maize.
Geographic distribution
Mexico
Biology and transmission
Sclerotia may overwinter in the soil or be planted mixed in with seeds. These germinate and develop many head-like structures (stromata) that release spores when the maize plants silk the following season.
Detection/indexing methods used at CIMMYT
- Physical inspection of seed
Treatment/control
- Not applicable.
Procedures followed in case of positive test used at CIMMYT
- Manual cleaning.
References and further reading
CAB International. 2007. Datasheet: Claviceps gigantea. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International.
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 17-18.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 68-69.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 55.
Maize doctor: http://maizedoctor.cimmyt.org/
Southern leaf blight, southern corn leaf blight, maydis leaf blight
Pathogen name
Cochliobolus heterostrophus (anamorph Bipolaris maydis
Other scientific names
Drechslera maydis (anamorph), Helminthosporium maydis (anamorph)
Importance
Low economic and phytosanitary importance.
Note
There are 3 physiological races of C. heterostrophus: O, T, and C. Race T and race C are pathogenic only to maize with Texas (T) and C male-sterile cytoplasm respectively. Race C is found only in China and is the least well-known.
Significance
The disease is most serious in areas with a warm and wet growing season. Race O mainly occurs in the tropics and subtropics, and its effects are usually minor, but yield losses as high as 68% have been recorded in Cameroon. An epidemic caused by race T in the USA in 1970 resulted in losses estimated at USD 1 billion, as 85% of the maize area was planted to maize with T male-sterile cytoplasm. However, no such maize is currently grown by farmers, so the significance of this race is now practically nil.
Symptoms
Symptoms of vary according to the causal race and host germplasm. Infections begin on lower leaves and progress up the plant.
Race O: lesions on leaves only. These are initially small and diamond-shaped, and elongate as they mature. Final lesions are rectangular (2-6 × 3-22 mm), restricted by leaf veins, and tan in color with brown borders. In severe infections, lesions may coalesce, producing complete burning of large areas of leaves; in these cases sugars may be diverted from the stalk for grain filling, increasing the risk of lodging.
Race T: on maize with T cytoplasm, lesions occur on all above-ground parts of the plant (including leaves, sheaths, stems and ears). Lesions are oval in shape and slightly larger (6-12 × 6-27 mm) than those caused by race O, with dark red-brown borders. In severe cases these may coalesce as above. Black mold may appear on ear. Seedlings from infected seed often wilt and die within 3-4 weeks. On maize with normal cytoplasm the pathogen may cause few small leaf spots.
 Maydis leaf blight caused by Cochliobolus heterostrophus (photo: CIMMYT) |
Hosts
Major host: maize.
Minor hosts: groundnut (Arachis hypogaea), soybean (Glycine max), sunflower (Helianthus annuus), rice (Oryza sativa), pearl millet (Pennisetum glaucum), pea (Pisum spp.), poplar (Populus deltoides), sorghum (Sorghum bicolor), wheat, cowpea (Vigna unguiculata), teosinte.
Wild host: Sudan grass (Sorghum sudanense).
Geographic distribution
Worldwide, predominantly tropics and subtropics. Regions with a warm (20-32°C) and damp growing season are most at risk.
Biology and transmission
Race T is seed-transmitted, while there is no evidence for seed transmission in race O. Both are seedborne, with high rates of infection. The fungus also overwinters on crop residues. At the onset of the subsequent growing season it begins producing spores, which are then wind and rain splash disseminated to freshly-planted maize in the vicinity.
Detection/indexing methods used at CIMMYT
- Freezing blotter test.
Treatment/control
- Resistance and appropriate fungicides.
Procedures followed in case of positive test used at CIMMYT
- Seed treatment with captan and thiabendazole.
References and further reading
CAB International. 2007. Datasheet: Cochliobolus heterostrophus. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International.
CIMMYT. Maize Doctor information sheet: Maydis leaf blight. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=251. Date accessed 10 April 2010
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 27-29.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 18-19.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 15.
Fusarium stalk rot, Fusarium ear rot, Fusarium kernel rot, Fusarium root rot
Pathogen name
Gibberella moniliformis (anamorph Fusarium verticilloides)
Other scientific names
Fusarium moniliforme (anamorph), Gibberella fujikuroi (teleomorph)
Importance
High economic importance. Seedborne.
Significance
The pathogen is very widespread, occurring throughout the maize-growing regions of the world; it is thought to be the most common maize ear-rot pathogen worldwide. Its impact depends on numerous factors, including host germplasm, climate and cultural practices, but both Fusarium stalk rot and Fusarium ear rot can cause significant crop damage and yield losses. G. moniliformis is further of concern as it produces mycotoxins (fumonisins) that are toxic to humans and livestock when heavily-infected grain is consumed, and cause economic losses.
Symptoms
The pathogen can cause a variety of disease symptoms, primarily stalk and ear rots.
Ear rot: damage occurs mainly on individual kernels or on limited areas of the ear (in contrast to Gibberella zeae). Infected kernels develop a cottony white, pale pink or lavender mycelium growth, or they may also display white streaks on the pericarp (also known as ‘starburst’ symptoms) and often germinate on the cob. Typically, infection occurs close to ear tips and is commonly associated with damage and injury caused by ear borers. Under severe infestation, the entire ear appears withered and is characterized by mycelium growth between kernels.
Stalk rot: symptoms are similar to those of G. zeae and also resemble those caused by Stenocarpella and Cephalosporium; positive identification often relies on microscopic examination of spores and fruiting structures. Infected plants typically wilt, leaves turn dull grayish-green and the lower stalk turns from dark green to straw-colored. Brown to black lesions develops near the lower stalk nodes, in which black perithecia may occur. The internal pith of the lower stem disintegrates and goes soft. When split open, stalks are generally discolored (appearing pinkish, reddish or brownish), and the phloem appears dark brown.
May also cause root rot and seedling blight.
Hosts
Broad host range: infects many crops, including maize, sorghum (Sorghum bicolor), sugarcane (Saccharum officinarum), wheat, cotton (Gossypium spp.), banana (Musa spp.), pineapple (Ananas comosus), and tomato (Solanum lycopersicum).
Geographic distribution
Worldwide; most common in drier, warmer climates.
Biology and transmission
Whether systemic seed transmission occurs is controversial; it has been shown in sterile soil but not in the field. However the pathogen is undoubtedly seedborne; infection levels of 100% have been reported. It can also survive on crop residues, forming spores that are wind and rain-splash disseminated to freshly-planted maize. Insects such as the European corn borer and the corn rootworm beetle may act as vectors, transferring spores between plants or causing injury that enables the fungus to infect the plant.
Detection/indexing methods used at CIMMYT
- Freezing blotter test.
Treatment/control
- Resistance, balanced soil fertility, lower plant populations.
Procedures followed in case of positive test used at CIMMYT
- Seed treatment with captan and thiabendazole.

Fusarium and Gibberella stalk rots (photo: CIMMYT) |

Gibberella ear rot |
References and further reading
CIMMYT. Maize Doctor information sheet: Fusarium and Gibberella stalk rot. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=266. Extended information sheet: Fusarium and Gibberella stalk rot. Online at:. Extended information sheet: Fusarium and Gibberella ear rot. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=235. Date accessed 10 April 2010
Leslie JF, Summerell BA. 2006. The Fusarium Laboratory Manual. Ames, IA: Blackwell Publishing. 388 pp.
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 13-15.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 46-47 and 66-67.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 6.
Pathogen name
Gibberella zeae (anamorph Fusarium graminearum)
Other scientific names
Fusarium graminearum group 2
Importance
High economic importance. Seedborne.
Significance
The pathogen is very widespread, occurring throughout the maize-growing regions of the world; it is the most common maize stalk-rot pathogen worldwide. Its impact depends on numerous factors, including host germplasm, climate and cultural practices, but both Gibberella stalk rot and Gibberella ear rot can cause significant crop damage and yield losses. G. zeae is further of concern as it produces mycotoxins that are toxic to humans and livestock when heavily-infected grain is consumed, and cause economic losses.
Symptoms
The pathogen can cause a variety of disease symptoms, primarily stalk and ear rots.
Ear rot: ear infection begins as white mycelium on the ear tips that gradually moves towards the base of the ear and turns a distinctive reddish-pink color in infected kernels. Early infection can result in entire ears being colonized by reddish-pink mycelium, extensive kernel rotting, and husks adhering tightly to ears. Bluish-black perithecia may also form on the husks.
Stalk rot: symptoms are similar to those of G. fujikori and also resemble those caused by Stenocarpella and Cephalosporium; positive identification often relies on microscopic examination of spores and fruiting structures. Infected plants typically wilt, leaves turn dull grayish-green and the lower stalk turns from dark green to straw-colored. Brown to black lesions develops near the lower stalk nodes, in which black perithecia may occur. The internal pith of the lower stem disintegrates and goes soft. When split open, stalks are generally discolored (appearing pinkish, reddish or brownish), and the phloem appears dark brown.
May also cause root rot and seedling blight.
 Fusarium and Gibberella ear rots (photo: CIMMYT) |
Hosts
Broad host range: in addiction to maize, infects other cereals including wheat, barley (Hordeum vulgare), oats (Avena sativa) and rye (Secale cereale); species of Lycopersicon, Pisum, Trifolium and Solanum; ornamental flowers.
Geographic distribution
Worldwide; most common in cooler and more humid climates.
Biology and transmission
The pathogen is seedborne: infection levels of 66% and survival over 13 years have been reported. Direct seed transmission has not been clearly demonstrated. Ascospores and conidia are produced both by perithecia that overwinter on crop residues, and by disease lesions during secondary disease cycles, and are wind and rain-splash disseminated. They may enter the plant through silks (causing ear rot), or at the stem, leaf sheaths or roots. The pathogen can also be spread by birds and insects.
Detection/indexing methods used at CIMMYT
- Freezing blotter test.
Treatment/control
- Resistance, balanced fertility, lower plant density.
Procedures followed in case of positive test used at CIMMYT
- Seed treatment with captan and thiabendazole.
References and further reading
CIMMYT. Maize Doctor information sheet: Fusarium and Gibberella stalk rot. [online] Available fro URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=266. Extended information sheet: Fusarium and Gibberella stalk rot. Online at:. Extended information sheet: Fusarium and Gibberella ear rot. [online] Available fro URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=236. Date accessed 10 April 2010
Leslie JF, Summerell BA. 2006. The Fusarium Laboratory Manual. Ames, IA: Blackwell Publishing. 388 pp.
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 60-62.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 46-47 and 66-67.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 5.
Anthracnose leaf blight and stalk rot
Pathogen name
Glomerella graminicola (anamorph Colletotrichum graminicola)
Importance
Moderate economic importance, low phytosanitary importance.
Significance
A serious problem in the USA (from the south east to the western corn belt), Europe, and India.
Important disease of sorghum, particularly in more humid regions and seasons.
Symptoms
The pathogen causes both a stalk rot and a leaf blight.
Stalk rot: narrow, elongated dark lesions appear on stem surface beginning when plants approach flowering. They are initially brown, turning shiny black and coalescing. Premature wilting of infected plants due to the complete destruction of pith tissue, with shredded vascular bundles turning dark brown.
Leaf blight: not reported to be of economic significance. Leaves show irregular, oval to elongated lesions with yellow to reddish-brown margins. These may coalesce. Acervuli develop in abundance on dead tissue.
May also cause root rot and seedling blight.
Increased disease severity has been associated with damage by the European corn borer and lesion nematode.
Hosts
Maize, sorghum (orghum bicolor), wheat, rye (Sorghum bicolor), barley (Hordeum vulgare), and other grasses.
Geographic distribution
Worldwide, particularly in warm, humid areas.
Biology and transmission
The pathogen is seed-transmitted and can survive for at least two years in seeds. It also survives on crop residues on the soil surface, releasing spores into the air. Conservation agriculture practices involving mulches reportedly increase the incidence of the disease.
Detection/indexing methods used at CIMMYT
- Freezing blotter test.
Treatment/control
- Resistance, crop rotation, balanced fertility.
Procedures followed in case of positive test used at CIMMYT
- Seed treatment with captan and thiabendazole.
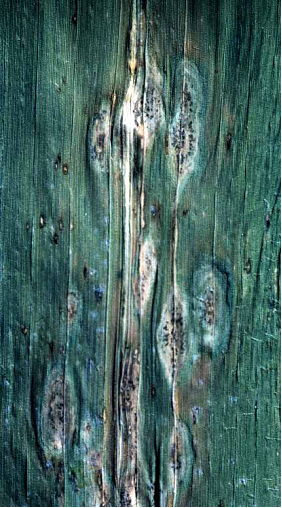
Anthracnose leaf blight (photo: CIMMYT) |

Anthracnose stalk rot (photo: CIMMYT) |
References and further reading
CAB International. 2007. Datasheet: Glomerella graminicola. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 3-4.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 54-55.
White DG. 1999. Compendium of Corn Diseases. Third Edition. St. Paul, MN: APS Press. 78 pp.
Nigrospora ear rot, cob rot of maize
Pathogen name
Khuskia oryzae (anamorph Nigrospora oryzae)
Importance
Occurs infrequently and is a weak parasite, therefore of low economic and phytosanitary importance.
Significance
The disease occurs infrequently, and causes minor losses of no more than a few percent. The ear rot it causes is of more importance than the stem rot.
Symptoms
On ears: Infected ears are chaffy and lightweight, with shredded cobs. Kernels are discolored and easily removed from the cob. Kernels have white streaks, with small black spore masses near the tips. These spore masses are also visible on cob tissues.
On stalks: superficial gray to black lesions, appearing as small blotches, which develop late in the season.
 Nigrospora ear rot (photo: CIMMYT) |
Hosts
Maize, rice (Oryza sativa), banana (Musa spp.).
Geographic distribution
Hungary, Poland, Romania, Serbia and Montenegro, India, Egypt, South Africa, the USA.
Biology and transmission
The pathogen is seed-transmitted, and also overwinters on crop residues.
Detection/indexing methods used at CIMMYT
- Agar plate test (Warham et al. 1996).
Treatment/control
- Resistance, control insects, balanced soil fertility.
Procedures followed in case of positive test used at CIMMYT
- Seed treatment with triazole fungicides.
References and further reading
CAB International. 2007. Datasheet: Khuskia oryzae. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International.
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 19-20.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 92-93.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. pp. 37 and 70.
Pathogen name
Peronosclerospora maydis
Other scientific names
Peronospora maydis, Sclerospora maydis
Significance
This is a serious disease in Indonesia, causing up to 40% crop losses. Losses were even estimated at 80 to 90% in some locations in 1964 and 1968. It has also been found in Australia.
Symptoms
On leaves, white to yellow streaks that later turn brown and necrotic. The fungus may become systemic and cause chlorosis in the upper leaves. Plants may be stunted and sterile. Downy growth may occur on the chlorotic streaks. Plants more than four weeks old are highly resistant to infection.
 Java downy mildew (photo: CIMMYT) |
Hosts
Maize.
Geographic distribution
Indonesia, Australia
Biology and transmission
Conidia from maize grown in the dry season are a major cause of infection for maize planted early in the rainy season. The conidia are always produced in the dark. They are wind and rain splash disseminated, but are fragile and cannot be disseminated more than a few hundred meters and do not remain viable for more than a few hours. Germination of the conidia requires free water to be available on the leaf surface, and infection occurs through stomata. The pathogen can also be seed-transmitted only before seeds are dried.
Detection/indexing methods used at CIMMYT
- Downy mildew detection test, with microscopic examination.
Treatment/control
- Seed treatment with metalaxyl. Resistance.
Procedures followed in case of positive test used at CIMMYT
- The seed lot is destroyed (the pathogen is quarantined in Mexico).
References and further reading
CAB International. 2007. Datasheet: Peronosclerospora maydis. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International
CIMMYT. Maize Doctor extended information sheet: Downy mildew. [online] Available fro URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=233. Date accessed 10 April 2010
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 18-19.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 4-7.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 73.
White DG. 1999. Compendium of Corn Diseases. Third Edition. St. Paul, MN: APS Press. 78 pp.
Pathogen name
Peronosclerospora philippinensis
Other scientific names
Sclerospora philippinensis
Importance
High economic and phytosanitary importance.
Significance
The pathogen is confined to South East Asia. It is a very serious problem in the Philippines, where yield losses to the disease often range between 15 and 40%. Yield losses in excess of 70% and even up to 100% have been recorded. At the national level, it was estimated to cause total losses of 8% in 1974/75. Losses of up to 60% have been recorded in India, but the disease is generally less severe there.
Symptoms
Systemic symptoms appear in the first true leaf as complete chlorosis or chlorotic stripes 9 days after planting. Local symptoms may appear from the 2-3 leaf stage until the tassels and silks are formed. In general infected leaves have long chlorotic streaks with downy growth of conidia and conidiophores. Infected plants may also have malformed tassels with reduced pollen production, and aborted ears with partial or complete sterility.
 Philippine downy mildew (photo: CIMMYT) |
Hosts
Maize and species belonging to the grass tribes of Andropogoneae and Maydeae.
Geographic distribution
The Philippines, China, India, Indonesia, Nepal, Pakistan, and Thailand.
Biology and transmission
The main source of inoculum is conidia produced in conidiophores that emerge from stomata. Infected weed hosts are a source of infection as well as other maize plants. The conidia are always produced at night. They are wind and rain splash disseminated, but are fragile and cannot be disseminated more than a few hundred meters and do not remain viable for more than a few hours. Germination of the conidia requires free water to be available on the leaf surface, and infection occurs through stomata. The pathogen can also be seed-transmitted and seedborne when seeds have moisture content above 36%.
Detection/indexing methods used at CIMMYT
- Downy mildew detection test, with microscopic examination.
Treatment/control
- Seed treatment with metalaxyl. Resistance, sanitation.
Procedures followed in case of positive test used at CIMMYT
- The seed lot is destroyed (the pathogen is quarantined in Mexico).
References and further reading
CAB International. 2007. Datasheet: Peronosclerospora philippinensis. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International
CIMMYT. Maize Doctor extended information sheet: Downy mildew. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=233. Date accessed 10 April 2010
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 22-24.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 4-7.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 73.
White DG. 1999. Compendium of Corn Diseases. Third Edition. St. Paul, MN: APS Press. 78 pp.
Pathogen name
Peronosclerospora sacchari
Other scientific names
Sclerospora sacchari
Importance
Moderate economic importance, high phytosanitary importance.
Significance
Sugarcane downy mildew is an important disease of maize in Australia and Asia, particularly in Taiwan where periodic epidemics have occurred. Yield losses range from 30 to 60%.
Symptoms
The disease initially appears as local small, round chlorotic spots on leaves. Later systemic infection causes yellow to white stripes on leaves. White, downy growth appears on both leaf surfaces, shanks, and husks. Tassels are improperly formed and ears are several, small, and poorly filled.
 Sugarcane downy mildew (photo: CIMMYT) |
Hosts
Maize, sugarcane (Saccharum officinarum).
Geographic distribution
Australia, Fiji, India, Japan, Nepal, New Guinea, the Philippines, Taiwan, and Thailand.
Biology and transmission
The fungus overseasons as mycelium in sugarcane. Conidia produced on diseased sugarcane are disseminated by wind or water-splash to maize leaves. The conidia produce germ tubes that penetrate the maize leaves through stomata. Tiny round lesions covered with ectophytic mycelia form at infection sites. The endophytic mycelium spreads intercellularly and the first streaks appear on young leaves. Once established the fungus produces large number of conidia that serve as secondary inoculum for maize and sugarcane seedlings. The pathogen can also be seed-transmitted when seed has moisture content above 20%.
Detection/indexing methods used at CIMMYT
- Downy mildew detection test, with microscopic examination.
Treatment/control
- Seed treatment with metalaxyl. Resistance, cultural practices, sanitation.
Procedures followed in case of positive test used at CIMMYT
- The seed lot is destroyed (the pathogen is quarantined in Mexico).
References and further reading
CAB International. 2007. Datasheet: Peronosclerospora sacchari. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International
CIMMYT. Maize Doctor information sheet: Sugarcane downy mildew. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=195. Extended information sheet: Downy mildew. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=233. Date accessed 10 April 2010
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 30-32.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 4-7.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 73.
White DG. 1999. Compendium of Corn Diseases. Third Edition. St. Paul, MN: APS Press. 78 pp.
Pathogen name
Peronosclerospora sorghi
Other scientific names
Sclerospora graminicola, Sclerospora sorghi
Importance
Moderate economic and low phytosanitary importance.
Significance
A serious disease in the tropics and subtropics. Severe outbreaks have occurred in India, Israel, Mexico, Nigeria, Texas, Thailand, and Venezuela. In Nigeria, yield losses as high as 90% have been reported.
Symptoms
On leaves, white stripes with a chlorotic area, always including the base of the leaf blade. There is usually a well-defined transverse between the diseased and healthy tissue, appearing further up the blade in each successively formed leaf (“half-leaf symptom”) until the whole blade is chlorotic. Leaves of infected plants tend to be narrower and more erect than those of healthy plants and shredding may occur. Downy growth may appear on both leaf surfaces. Tassels may show phyllody; ears barren or only a few seeds produced.
 Sorghum downy mildew (photo: CIMMYT) |
Hosts
Major hosts: maize, sorghum (Sorghum bicolor), Sudan grass (Sorghum sudanense).
Minor hosts: Andropogon sorghi, Panicum trypheron, pearl millet (Pennisetum glaucum), Johnson grass (Sorghum halepense), teosinte.
Geographic distribution
Worldwide.
Biology and transmission
Both conidia and oospores are sources of disease inoculum.
The oospores can survive in the soil for several seasons. At the onset of the growing cycle they germinate in response to root exudates from susceptible maize seedlings. The germ tube infects the underground sections of maize plants leading to characteristic symptoms of systemic infection including extensive chlorosis and stunted growth. No infection by oospores occurs if the seedlings emerge in cool soils. Conidia are produced profusely during the growing season on the leaves and are disseminated by the wind, providing secondary inoculum, which can induce systemic infection in plants up to four weeks old.Oospores are produced in large numbers only on systemically infected plants.
The pathogen can also be seedborne and seed-transmitted in cases where seeds are fresh and have high moisture content.
Detection/indexing methods used at CIMMYT
- Downy mildew detection test, with microscopic examination.
Treatment/control
- Seed treatment with metalaxyl. Resistance, sanitation, rotation.
Procedures followed in case of positive test used at CIMMYT
Seed treatment with metalaxyl. Resistance, cultural practices.
References and further reading
CAB International. 2007. Datasheet: Peronosclerospora sorghi. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International.
CIMMYT. Maize Doctor information sheet: Sorghum downy mildew. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=284. Extended information sheet: Downy mildew. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=233. Date accessed 10 April 2010
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 25-27.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 4-7.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 73.
White DG. 1999. Compendium of Corn Diseases. Third Edition. St. Paul, MN: APS Press. 78 pp.
Pathogen name
Sclerophthora rayssiae var. zeae
Importance
Low economic importance, moderate phytosanitary importance.
Significance
The pathogen was first observed in India in 1962, where it is a common and serious disease, with yield losses ranging from 20 to 90%. It tends to be most severe in areas with 100-200 cm annual rainfall, declining in severity as rainfall decreases.
Symptoms
Chlorotic or yellow stripes on leaf, initially narrow, becoming red to purple, with well-defined margins and delimited by the veins. Lateral development of lesions causes severe striping and blotching. Downy growth of sporangia occurs on both leaf surfaces. Seed set may be reduced.
 Brown stripe downy mildew (photo: CIMMYT) |
Hosts
Maize, hairy crabgrass (Digitaria sanguinalis).
Geographic distribution
India, Nepal, Pakistan and Thailand.
Biology and transmission
The pathogen survives as oospores in infected debris in soil. Upon germination, the oospore produces a sporangiophore bearing a sporangium that liberates between four and eight zoospores. Soil temperatures of 28-32°C favor disease development.
Secondary spread is by sporangia. Sporangial production, germination and infection require a film of moisture for at least 12 hours.
The pathogen can also be seedborne and seed-transmitted when seeds have moisture content higher than 14%.
Detection/indexing methods used at CIMMYT
- Downy mildew detection test, with microscopic examination.p>
Treatment/control
- Resistance, rotation, soil fertility.
Procedures in case of positive test used at CIMMYT
- The seed lot is destroyed (the pathogen is quarantined in Mexico).
References and further reading
CAB International. 2007. Datasheet: Sclerophthora rayssiae var. zeae. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International
CIMMYT. Maize Doctor information sheet: Downy mildew. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=233. Date accessed 10 April 2010
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 8-10.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 4-7.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 73.
White DG. 1999. Compendium of Corn Diseases. Third Edition. St. Paul, MN: APS Press. 78 pp.
Northern corn leaf blight, maize leaf blight, turcicum leaf blight
Pathogen name
Setosphaeria turcica (anamorph Exserohilum turcicum)
Other scientific names
Bipolaris turcica (anamorph), Drechslera turcica (anamorph), Helminthosporium turcicum (anamorph)
Importance
The disease can be very serious and must be controlled in the field during genebank operations.
Significance
The disease is common, but usually causes minor losses. Symptoms and attendant losses are most severe when infection occurs prior to or at silking. However, it is a major constraint to production, causing severe outbreaks, in many maize-growing regions worldwide with a growing season characterized by high humidity (with periods of extended dew or leaf wetness) and moderate temperatures (17 to 27°C). Yield losses as high as 70% have been recorded, although losses typically range from 15 to 30%.
Symptoms
The earliest symptoms are most easily recognized: small, oval, water-soaked spots on leaves. These grow into elongated, spindle-shaped or elliptical necrotic lesions, 3-15cm long. Lesions develop distinct dark areas as they mature associated with fungal sporulation; these may appear as concentric rings. Lesions typically first appear on lower leaves, spreading to upper leaves and the ear sheaths as the crop matures. Under severe infection, lesions may coalesce, blighting the entire leaf. Lesions may vary slightly depending on the resistance status of the host; on some hybrids with resistance genes symptoms may include long, chlorotic streaks that can be confused with other diseases such as Stewart’s Wilt.
 Northern corn leaf blight (photo: CIMMYT) |
Hosts
Maize, sorghum (Sorghum bicolor), pearl millet (Pennisetum glaucum).
Geographic distribution
Worldwide, predominantly subtropical to temperate regions.p>
Biology and transmission
The pathogen is seedborne on the seed surface. It also overwinters as mycelium and chlamydospores in infected crop residues and soil. At the onset of the subsequent season, the fungi begin to sporulate in response to higher temperatures and humidity. Spores (conidia) are then disseminated by wind and rain-splash to freshly planted maize. Conidia can be carried vast distances in the wind. They germinate in temperatures ranging from 17 to 27°C and during periods of extended leaf wetness (6 to 18 hours), infecting host tissue. Secondary cycles of disease occur where conidia produced in disease lesions are disseminated within the crop and to other fields by rain-splash and wind.
Detection/indexing methods used at CIMMYT
- Freezing blotter test.
Treatment/control
- Resistance; in seed fields appropriate fungicides.
Procedures followed in case of positive test used at CIMMYT
- Seed treatment with captan and thiabendazole.
References and further reading
CIMMYT. Maize Doctor information sheet: Turcicum leaf blight. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=205. Extended information sheet: Turcicum leaf blight. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=247. Date accessed 10 April 2010
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 96-97.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 16-17.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 24.
White DG. 1999. Compendium of Corn Diseases. Third Edition. St. Paul, MN: APS Press. 78 pp.
Diplodia ear rot, Diplodia stalk rot
Scientific name
Stenocarpella maydis and S. macrospora
Other scientific names
Diplodia maydis, Diplodia zeae, Diplodia macrospora
Importance
High economic and phytosanitary importance.
Significance
Stenocarpella maydis and S. macrospora cause both Stenocarpella ear rot and Stenocarpella stalk rot. Stenocarpella stalk rot is common in most maize growing regions globally, caused by S. maydis in cool, humid temperate areas, and by S. macrospora in warm, humid areas. Stenocarpella ear rots caused by both species are common in hot, humid maize growing regions. Yield losses are generally minor but can be considerable. Infection of the ears by Stenocarpella species can also result in the production of mycotoxins (including diplosporin).
Symptoms
Ear rot: Infection just after flowering results in characteristic irregular bleached areas on husks. The fungus is visible as coarse, white to grayish-brown mycelial growth over the husks and kernels. Symptoms typically appear first at the base of the ear. The ear may be shrunken and turn grayish brown, and the infected kernels appear “glued” to the husk by white fungal mycelium. Infected kernels are dull gray to brown. Infected ears may be very light and totally rotted. Late in the season, abundant, black pycnidia may form on kernel and ear tissues, including husks. Late-infected ears show no external symptoms, but when ears are broken and grains removed, a white mould is commonly found growing between the grains.
Stalk rot: the pith of basal internodes becomes brown and the stalk may break easily. Later in the season, the most conspicuous symptom is the abundant formation of pycnidia on the surface of infected stalk tissue.
Infected seed gives rise to pre-emergence death in cold soils or blighted seedlings in warmer soils.
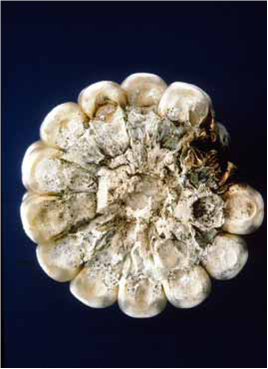 Stenocarpella ear rot (photo: CIMMYT) |
Hosts
Maize.
Geographic distribution
Worldwide.
Biology and transmission
Seed transmission is an important source of inoculum. The fungus also overwinters as pycnidia and mycelium on stalk residues buried or left on the soil surface. Under cool and humid conditions in the case of S. maydis, and warmer conditions in the case of S. macrospore, these produce conidia, which are disseminated by wind, rain-splash, and probably insects. Ear rot is caused by infection through silks, at the base of the ear or through ear injuries caused by birds and insects. Stalk rot is typically caused by infection through the crown, mesocotyl, roots, or, occasionally, the nodes between the crown and ear. Stalks are then invaded. Conidia of S. maydis rapidly lose their viability at high temperatures and on exposure to sunlight.
Detection/indexing methods used at CIMMYT
- Freezing blotter test.
- Agar plate test (Warham et al. 1996).
Treatment/control
- Seed treatments are fairly effective in controlling seedling blight, but once the fungus is established in the soil, crop rotation is necessary to eliminate it.
Procedures followed in case of positive test used at CIMMYT
- Seed treatment with captan and thiabendazole.
References and further reading
CIMMYT. Maize Doctor information sheet: Stenocarpella ear rot. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=216. Extended information sheet: Stenocarpella ear rot. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=248. Date accessed 10 April 2010
CIMMYT. Maize Doctor information sheet: Stenocarpella ear rot. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=196. Extended information sheet: Stenocarpella ear rot. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=245. Date accessed 10 April 2010
EPPO. Data Sheets on Quarantine Pests: Stenocarpella macrospora and Stenocarpella maydis. [online] Available from URL: http://www.eppo.org/QUARANTINE/fungi/Stenocarpella_macrospora/DIPDSP_ds.pdf. Date accessed 10 April 2010
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 11-13.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 60-61 and 82-83.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 53 and 70.
White DG. 1999. Compendium of Corn Diseases. Third Edition. St. Paul, MN: APS Press. 78 pp.
Pathogen name
Ustilago maydis
Other scientific names
Ustilago zeae
Importance
Moderate economic and low phytosanitary importance.
Significance
Common smut has been documented in most maize-growing regions, but rarely causes economic losses. The disease is more severe in humid, temperate environments than in hot, humid, tropical lowlands. In Mexico, the galls formed on maize ears by Ustilago maydis are consumed as a delicacy known as huitlacoche.
Symptoms
Characteristic galls form on most aboveground parts of the plant, including ears (replacing kernels), stalks, tassels and leaves. Galls are semi-fleshy and initially light in color (greenish or grayish white) and firm with a shiny periderm. As the galls mature they turn a dark grey color (except those on the leaves), and contain masses of powdery dark gray to black spores; they eventually disintegrate and release the spores. The galls that form at the ear tip are most common and distinctive and can reach up to 15cm in diameter. Galls on the leaf typically form on the midrib and distort the leaf, but rarely exceed 1.5cm in diameter. The disease is most severe in young plants as infection of the meristematic tissues can result in stunting or plant death.
 Common smut caused by Ustilago maydis (photo: CIMMYT) |
Hosts
Maize, teosinte.
Geographic distribution
Worldwide.
Biology and transmission
Teliospores are released from ruptured galls and overwinter in the soil, where they remain viable for several years. Under favorable environmental conditions, teliospores germinate to form haploid sporidia. Sporidia of compatible mating groups fuse to form diploid mycelia that can infect maize plants. Teliospores can also infect maize plants directly. Dissemination is by wind. Infection usually occurs through injuries or, in the case of ears, through the silks. Host cells around the infective hyphae expand, leading to the production of galls. In ears these replace kernels. The pathogen is not seed-transmitted, but may be seedborne as healthy kernels from infected ears can be coated in teliospores.
Detection/indexing methods used at CIMMYT
- Seed washing and filtration test.
Treatment/control
- Seed treatment is possible.
- The most effective control of the disease can be obtained growing resistant maize lines and hybrids.
Procedures followed in case of positive test used at CIMMYT
- Seed treatment with captan and thiabendazole.
References and further reading
CIMMYT. Maize Doctor information sheet: Common smut. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=219. Extended information sheet: Common smut. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=230. Date accessed 10 April 2010
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 54-55.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 76-77.
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. p. 62.
White DG. 1999. Compendium of Corn Diseases. Third Edition. St. Paul, MN: APS Press. 78 pp.




