CGKB News and events Collecting
Collecting plant genetic diversity: Technical guidelines. 2011 update
Contact person: Elizabeth Goldberg, Bioversity, Italy

Alcohol-sterilized shoot tip being placed in a vial of growth medium for transport. (Photo: V. Pence.) |
|
|
Contents: |
Introduction
In 1995, Bioversity International, (then the International Plant Genetic Resources Institute-IPGRI), in association with FAO, IUCN and UNEP, produced “Collecting Plant Genetic Diversity; Technical Guidelines,” edited by L. Guarino, V. Ramanatha Rao and R. Reid and published by CABI.

Michael Way, of Kew, collecting Ephedra andina for the Millennium Seed Bank. |
This online resource updates the 1995 classic reference and provides two new topics. The Collecting Manual, as it came to be known, covers all the steps in collecting. CABI has generously given permission for the chapters from the original publication to be accessed online alongside the updates. Two of the original editors, L. Guarino and V. Ramanatha Rao, have led this updating activity in collaboration with Bioversity and a team of more than 40 experts from around the world.
This resource guides new and experienced collectors on how to sample, collect and preserve a wealth of genetic resources – not only crop plants and trees but also wild species, symbiotic bacteria and fungi, pollen and even DNA. It also gives advice on related topics such as ecogeographic surveys, the use of geographic information systems and other data management tools, data recording, taxonomic identification and on the legal issues involved in collecting genetic resources. In addition to synthesizing new knowledge, each chapter provides references--many of them available online--and complementary internet resources. Users are encouraged to comment or update content on forms provided.
A zip file of all the updated chapters in one folder can be downloaded here (13.9 MB).
Chapters 30 to 39 are case studies from the 1995 Collecting Manual.
A zip file of all the case studies in one folder can be downloaded here (2.1 MB).
References and further reading
Bioversity Internatioanl 2013 [online] Bioversity collecting mission database. Available from http://www.bioversityinternational.org/collecting_missions.html Date accessed: 5 March 2013.
Guarino L, Ramanatha Rao V, Reid R, editors. 1995. Collecting plant genetic diversity: Technical guidelines. International Plant Genetic Resources Institute (IPGRI), Rome, Italy; Plant Production and Protection Division, FAO, Rome, Italy; World Conservation Union (IUCN), Gland, Switzerland; CABI, Wallingford, UK. 748 pp. ISBN: 0-85198-964-0
Gyllensten U. 1984. A neglected heritage. Documentary film about plant genetic resources. Produced by Ulf Gyllensten in 1984 for the International Board for Plant Genetic Resources (IBPGR) in cooperation with the Nordic Gene Bank. Watch the video here.
Smith RD, Dickie JB, Linington SH, Pritchard HW, Probert RJ, editors. 2003. Seed conservation: turning science into practice. London: The Royal Botanic Gardens, Kew. 1023 pp. Available online (accessed 17 October 2011): www.kew.org/science-research-data/kew-in-depth/msbp/publications-data-resources/technical-resources/seed-conservation-science-practice/index.htm
SGRP. 2011 [online] Crop collecting missions - repository of reports. Available from http://www.central-repository.cgiar.org/crop_collecting_missions.html Date accessed: 15 December 2011.
SGRP. 2011 [online] IBPGR/IPGRI collected sample database. Available from http://singer.cgiar.org/index.jsp?page=coll-sample-data. Date accessed: 15 December 2011.
Thormann I, Gaisberger H, Mattei F, Snook L, Arnaud E. 2012. Digitization and online availability of original collecting mission data to improve data quality and enhance the conservation and use of plant genetic resources. Genetic Resources and Crop Evolution. 59:5 635-644. DOI 10.1007/s10722-012-9804-z. http://link.springer.com/article/10.1007/s10722-012-9804-z?null.
Acknowledgements: Bioversity would like to thank the following persons for their generous contributions to this 2011 update. Luigi Guarino and V. Ramanatha Rao for their role as scientific editors and peer reviewers; Elizabeth Goldberg, production manager and editor; Kathleen Sheridan, copy editor; Imke Thormann and Geert Claessens, web editors; Vanessa Alam, documentation; 40 authors from 26 organizations (see authors in chapters at links below). Bioversity provides a very special thanks to CABI for generously allowing the original chapters to be accessed online, together with the update on this portal.
Citation to this web page
Guarino L, Ramanatha Rao V, Goldberg E, editors. 2011. Collecting Plant Genetic Diversity: Technical Guidelines - 2011 Update. Bioversity International, Rome, Italy. ISBN 978- 92-9043- 922- 6. Available online: http://cropgenebank.sgrp.cgiar.org/index.php?option=com_content&view=article&id=390&Itemid=557
The editors invite your comments on the Collecting Procedures pages by using the Comments feature at the bottom of this page or at the bottom of each of the chapters.
Chapter 1. A brief history of plant germplasm collecting
| image |
Abstract
PDF 2011 + PDF1995
Ackowledgements
References and further reading
Chapter 11: Aids to taxonomic identification
N. Maxted
School of Biological Sciences, University of Birmingham, UK
E-mail: nigel.maxted(at)dial.pipex.com
|
2011 version |
1995 version |
||
|
Open the full chapter in PDF format by clicking on the icon above. |
|||
This chapter is a synthesis of new knowledge, procedures, best practices and references for collecting plant diversity since the publication of the 1995 volume Collecting Plant Diversity; Technical Guidelines, edited by Luigi Guarino, V. Ramanatha Rao and Robert Reid, and published by CAB International on behalf of the International Plant Genetic Resources Institute (IPGRI) (now Bioversity International), the Food and Agriculture Organization of the United Nations (FAO), the World Conservation Union (IUCN) and the United Nations Environment Programme (UNEP). The original text for Chapter 11: Aids to Taxanomic Identification, authored by N. Maxted, has been made available online courtesy of CABI. The 2011 update of the Technical Guidelines, edited by L. Guarino, V. Ramanatha Rao and E. Goldberg, has been made available courtesy of Bioversity International.
Please send any comments on this chapter using the Comments feature at the bottom of this page. If you wish to contribute new content or references on the subject please do so here.
Back to list of chapters on collecting
Internet resources for this chapter
|
Practicing characterization using a color chart at a training course (photo: Bioversity) |
Abstract
To effectively conserve and utilize species, the conservationist must be able to distinguish and identify them correctly. The process of specimen identification or determination involves two steps: the decision as to which taxon (e.g., genus, species or subspecies) the specimen represents and what the “accepted” name for it is, if more than one name has been used for that taxon. The first step is achieved through some form of key or identification aid, while the second involves finding the accepted name for the taxon. Taxa are still primarily identified using single-access dichotomous keys, although multi-access keys and illustrations or photographs can be used.
In this updated chapter on taxonomic identification, progress has been reviewed in six key areas: (1) botanical glossaries, (2) combining text and illustrations for identification, (3) interactive identification, (4) conservation field guides, (5) barcode identification and (6) accepted names. Although there have been advances in identification science in recent years, poor taxonomic training and lack of user-friendly identification aids remain an inhibiting factor in the conservation and use of plant genetic resources.
Introduction
The original chapter starts with the statement that “Increasing attention is being focused on the collection and conservation of wild species” but since that text was written, the focus of conservation of plant genetic resources has turned even more towards conserving wild species, which has meant identifying greater numbers of specimens. Crop diversity is already largely conserved, at least for the major crops, and seed is conserved ex situ in genebanks. The second report of the Food and Agriculture Organization of the United Nations (FAO) on the state of the world’s plant genetic resources for food and agriculture (SoW2) reports that 90% of genebank accessions are of crop material (FAO 2010); however, there is growing recognition that crop wild relatives (CWR) are an under-exploited resource (Maxted and Kell 2009).
Feuillet et al. (2008) question the ability of breeders to increase or simply sustain crop yield and quality in the face of growing and dynamic biotic and abiotic threats. They suggest that the increasing ease of gene discovery, the development of enabling genetic and breeding techniques and a better understanding of the previous limitations on exotic germplasm make CWR the obvious choice for meeting contemporary demands for food security. This is reflected in the clear increase in the number of CWR collections between 1996 and 2009 (FAO 2010).
Given the changing focus of germplasm collection from crop to wild species (FAO 2010), the growing need to establish genetic reserves for in situ CWR conservation, and the demise of teaching taxonomy (House of Lords 2002), the need for skills in plant identification has never been greater. It is probable that lack of identification skills remains a fundamental limitation to plant genetic resource conservation and use. How can we conserve or exploit plant diversity if we cannot recognise it in the field?
Current status
With the imperative to conserve and use wild biodiversity, it might be expected that the taxonomic community would respond with a new generation of identification tools that avoid the pitfalls of classical botanical terminology and the arcane practice of using keys. But today, field identification is still largely based on the use of a hand lens and dichotomous keys: there have been no fundamental changes since 1995 (and even in fact 1895) when the original texts were written. However, some new developments that offer opportunities and challenges are noted below.
Botanical glossaries
One of the major limitations to using traditional keys in the field is the extent of the botanical terminology used. By definition, dichotomous keys are based on variations in gross morphological characteristics, and the range of characteristics used to describe the major groups of taxa will vary substantially between groups because their basic morphology is different. The suite of characteristics used to describe cereals will be very different from those describing legumes, and they will both be very different from wild cucurbit species.
Each major group of plants has its own associated descriptive terminology, but today there are very few botanists who have sufficient skills (i.e., knowledge of the general and group-specific terminology) to identify any plant species encountered in the field. Almost in recognition of this fact, there have been several botanical glossaries published in recent years: notably, Hickey and King (2000), which is split between a written glossary and a series of annotated illustrations of plant parts; Harris and Woolf Harris (2001) and Beentje (2010), which are both a glossary with illustrations throughout. It is advisable for any plant conservationist collecting from the wild, surveying a genetic reserve, or re-identifying grown-out specimens to keep one of these glossaries close to hand.
Combining text and illustrations for identification
When describing the various ways to identify biodiversity, many of the taxonomic text books recommend a combination of text and images in keys to avoid reliance on botanical terminology alone. Figure 1 shows a lateral multi-access to British grasses, where the illustrations at the head of each column clearly show the characteristic described. Although this remains an efficient concept, it has been taken up by surprisingly few taxonomists. No major Floras routinely use illustrations within their keys, and the few examples where textual keys and illustrations are combined are often associated with specialist publications, such as Aids to Identification in Difficult Groups of Animals and Plants (AIDGAP) (www.field-studies-council.org/publications/aidgaptesters.asp). The AIDGAP project is producing a range of books designed to provide clearly written and illustrated guides to enable the non-specialist to identify specimens in the field and lab—techniques that could clearly be usefully applied in the context of plant genetic resource conservation.
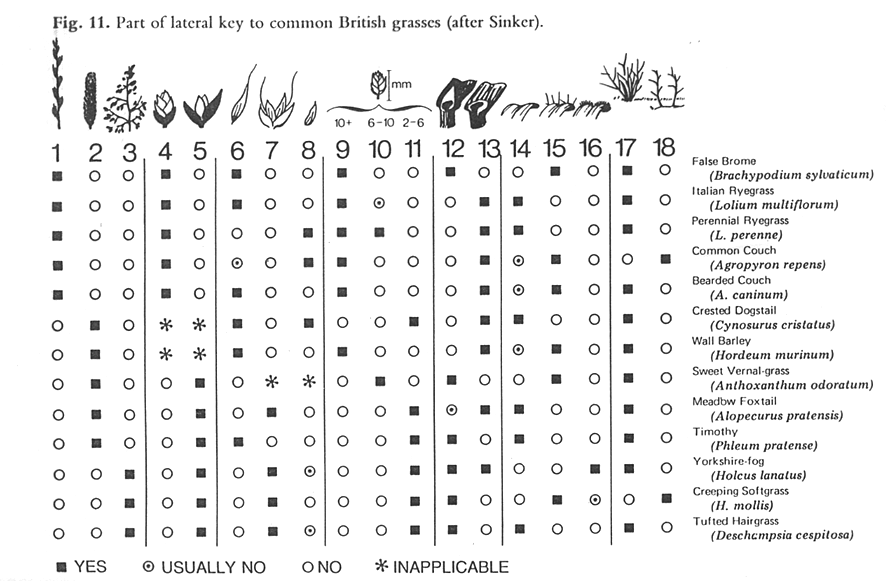 |
|
Source: Pankhurst (1978) Figure 11.1. Part of a lateral key to common British grasses. |
Interactive identification
The nature of the traditional dichotomous keys means that to identify a specimen, the user must follow a specific and structured sequence of questions until the identification is finally made. However, the user might not be able to proceed past the first couplet because neither of the leads provided in the first couplet fits the specimen (e.g., the first couplet might ask a question about seed characteristics but no seeds are present on the specimen) or neither of the two alternative descriptions in a couplet appears to describe a specimen accurately.
Multi-access keys were developed to overcome some of the problems associated with single-access keys. A multi-access key does not force the user to go through the character set in a specific preordained sequence; rather, it allows the user to ignore particular characters and still obtain an identification. For example, if the specimen lacks seeds, the identification can be based on vegetative and flower characteristics alone.
Computers are now commonly used for multi-access identification—a process that is referred to as “interactive identification” because the user interacts with the identification program and data set to obtain the identification. Given that identification is often an unconscious process, interactive identification keys should try to mimic the way we identify objects naturally and help reduce the user's frustration when the process becomes more explicit. Stevenson et al. (2003) suggested that these keys should (1) provide training tools and games to let people become familiar with the “cast of characters” slowly instead of being overwhelmed and confused by having to learn a lot of new things at once, (2) work to reduce the time necessary to identify a species by choosing likely possibilities from a line-up approach and (3) suggest further queries that will aid in making the final positive identification. The interactive identification program holds a matrix of taxa against characters, possibly including both text and images, and the user enters attributes (character-state values) of the specimen to be identified. The program eliminates taxa whose attributes do not match those of the specimen. This process is continued until only one taxon remains, giving a provisional identification. As with all identifications, the specimen may then be checked against a description, images or already named specimens to confirm the identification.
There are several interactive identification programs now available. For example, Linnaeus II (www.eti.uva.nl/products/linnaeus.php) is a commercial program available from the University of Amsterdam, the Netherlands. It is free, if you register as a data provider. Linnaeus II supports the creation of taxonomic databases, optimizes the construction of easy-to-use identification keys, expedites the display and comparison of distribution patterns, and promotes the use of taxonomic data for biodiversity studies. There are three modules of Linnaeus II: the “Builder” to manage your data and to create an information system, the “Runtime” engine to publish completed information systems on CD-ROM/DVD-ROM, and the “Web Publisher” to publish your completed project as a website.
A second example is Lucid (www.lucidcentral.com/), which is available from the Centre for Biological Information Technology, University of Queensland, Australia. Similar to Linnaeus II, the Lucid system consists of a number of inter-related products that assist with the creation and use of keys (in any language) for any group of organisms. Lucid software is a special type of expert system, specifically designed for identification and diagnostic purposes, which enables expert knowledge to be “cloned” and distributed to a wide audience via CD or the internet. Lucid-based keys are accessed via the Lucid Player, which provides the interface for users to load and interact with the Lucid keys, using text, images, videos and sounds to help select those taxonomic, diagnostic or other features that best describe the particular case being investigated. The key works by elimination: as the user selects character states, the program eliminates the taxa that do not possess that state. This is repeated until a single taxon remains and the identification is achieved. Once a specimen has been identified, the user is provided with a full range of multimedia fact sheets: descriptions, conservation notes, sub-keys for infra-specific taxa or links to websites for further information or recommendations. Lucid keys can be built in various languages and use terminology familiar to the user, allowing the package to be used internationally and across a wide range of capabilities.
Both the Linnaeus II and Lucid 3 identification keys are being increasingly used by a wide range of people, from high school and university students to taxonomists, quarantine identifiers, biodiversity scientists and conservation managers. As an example of an interactive key, an exemplar data set for African Vigna (Maxted et al. 2004) is included in Lucid 3 and can be downloaded from the Lucid website (http://keys.lucidcentral.org/keys/African_Vigna/default.htm).
Conservation field guides
Anyone working in field conservation is quickly forced to address the identification problem: how do I identify all the species I am confronted with in the field and wish to conserve? The majority of identification aids produced by taxonomists are, by far, conventional dichotomous keys that need someone in the field team with the appropriate skills to use these highly technical keys. The lack of formal taxonomic teaching in the developing world and the demise of such teaching in the developed world means that increasingly people with the necessary skills are not available. This has led to significant problems in field collection or survey activities for conservation projects (notably with several CWR conservation projects funded by the Global Environment Facility), not to mention individual national activities around plant genetic resources. For example, the field botanists employed by the project “Conservation and Sustainable Use of Dryland Agrobiodiversity in Jordan, Lebanon, the Palestinian Authority and Syria” were contracted to undertake such field survey work but had little if any prior experience using traditional identification aids, which seriously limited project activities (Al-Atawneh et al. 2009). In this example, even when specific identification training was provided to the staff, few were sufficiently confident at the end of the course to go into the field and use conventional dichotomous keys to identify the project taxa. It forced the project team to rethink how field identification might best be achieved. The proposed solution was to create a series of user-friendly conservation field guides, the first of which was recently published by the International Center for Agricultural Research in the Dry Areas (ICARDA) for Medicago species (Al-Atawneh et al. 2009).
These conservation field guides contain a combination of simple traditional keys, descriptions, illustrations, photographs and interactive identification keys. A comparison of the basic difference between identification using a standard taxonomic Flora and conservation field guides is made in table 11.1. Using descriptions or illustrations for identification involves matching a particular specimen with the characteristics of known species that are drawn, photographed or described in the guide. Keys help users focus their search on a section of the guide where the number of choices is relatively small. Then, by scanning the species illustrations, a tentative identification can be made. The best guides are the ones that give references to similar species in each species account. Users can make direct comparisons with these to increase the confidence of a positive identification. Sometimes, one single taxon-specific character among all of those given is enough to identify the species (e.g., a leaf, a flower, a twig, a fruit, or a piece of bark for trees, or even a specific habitat).
Table 11.1. Comparison of floras and field guides
|
Flora |
Conservation Field Guide |
|
Designed to be used primarily in herbaria by taxonomists (cumbersome to take on field trips) |
Designed to be used primarily in the field by field botanists (robust structure suitable for field use) |
|
Rarely planned for use by non-experts |
Planned for use by non-experts, often commercial |
|
Heavy and expensive, occupying much shelf space |
Ideally in one portable volume |
|
Emphasizing formal taxonomy, with lists of synonyms and specimen citations |
Emphasizing information required to identify the plants |
|
Resolving issues over taxonomic limits, accepted plant names and synonyms |
Relying on other works (usually a Flora or monograph) to define accepted plant names |
|
Relying on traditional dichotomous keys and full descriptions for identification |
Using a wide range of identification aids |
|
Generally including all vascular plants in a geographic area |
Typically restricted to a narrower subset of all species than a flora (for example, crop wild relatives) |
|
Focus on precise botanical characters, even if obscure, but offering precision |
Focus on easily observed field characters |
|
Often take decades to write, lack practical information on conservation status, cultivation or usage |
Usually prepared relatively quickly and containing additional information like conservation status, cultivation or usage |
|
Few illustrations or pictures |
Most species illustrated or photographs included |
Source: Adapted from Lawrence and Hawthorn (2006).
What makes a “conservation” field guide? Many field guides have a focus on identification alone but a “conservation” field guide should enhance the conservation value of the guide by including information on current conservation status and threat assessment in the centre of diversity of the genus, along with future conservation requirements. Further, for conservationists of plant genetic resources, there is an essential link between conservation and use; therefore, for each species the guide would include additional information on the actual and potential use value of the species. There is no precise universal format for such a guide, especially as they may focus on a particular habitat, region or taxon, but they commonly present a taxonomic background, morphological descriptions, habitats, behaviour, ecology, distribution maps, uses, conservation notes and simple dichotomous keys suitable for field use, possibly annotated with line drawings, photographs, or paintings. To specifically address the problem of non-expert identification, the printed guide will ideally be accompanied by a CD with an interactive identification system to the taxa covered in the guide.
A good guide is one that is widely used by the target audience. It should be accurate, attractive, relevant, affordable and available; but it should also convey good-quality information that actually improves the knowledge of the user. Lawrence and Hawthorne (2006) define this as the guide being usable: users can find, understand and apply the information contained in it to meet their needs. Lawrence and Hawthorne stress that the authors of such guides need to involve a range of experts (who may include local experts with traditional knowledge), as well as potential users, in planning and researching the guide’s style and content so that the final product is the result of collaboration and not dictation.
A useful development would an on-line list of available conservation field guides. Further, such a site could be a location from which on-line conservation field guides could be downloaded, possibly similar to the eFloras website where copies of some standard Floras can be downloaded (www.efloras.org/index.aspx). This would mean that, as the resource grew, anyone planning conservation could look on-line at the site and download the identification tool they require for the taxa or region in which they are planning to undertake active conservation.
Barcode identification
In recent years, the rapid advance of molecular techniques has opened the possibility of using DNA samples for specimen identification (Kress et al. 2005), and so-called DNA barcoding uses a short genetic marker in an organism's DNA to identify it as belonging to a particular species. While this would not have a field application (at least at this time), it does mean that if a specimen cannot be identified using more conventional means, a DNA sample could be taken and compared against a DNA reference collection. Specimens can also be identified from plant parts (e.g., even leaves or roots) when the flowers or fruit normally used for identification are unavailable.
For the system to work, a desirable locus for DNA barcoding needs to be agreed upon, so that large databases of sequences for that locus can be developed for each plant species. The Plant Working Group for the Consortium for the Barcode of Life (CBOL) (2009) have suggested the concatenation of the rbcL and matK chloroplast genes as the locus for plants. The DNA sequences would be stored in a DNA sequence database, like GenBank, but there would be a need to link DNA sequences to vouchered specimens to ensure that the sequences are grounded to a named specimen of the taxon (Miller 2007). Although DNA barcoding has its critics as a technique and there are still technical problems that remain with its practical implementation, it seems likely to be a technique of growing importance in future years.
Accepted names
Identification is a two-stage process. First, the decision must be made as to which taxon (e.g., genus, species or variety) the specimen represents and, second, what the “accepted” name for that taxon is. Taxonomists’ views on the “accepted” name for a taxon will continue to vary as views on how taxa are related change with new taxonomic evidence. While this will occasionally result in taxon name changes, in recent years there has been significant progress toward the production of more stable lists of accepted species. The Convention on Biological Diversity (CBD) recognized the need for a stable list of taxa in the Global Strategy for Plant Conservation 2011-2020 (CBD 2010) under Objective I: “Plant diversity is well understood, documented and recognized”. The first specific action (Target 1) is an online Flora of all known plants.
Since the 1980s many multi-institutional database projects have been collating biodiversity information for either specific taxonomic groups or geographical regions. For example, the International Legume Database and Information Service (ILDIS) (http://ildis.org/) was one of the first monographic databases to be established for the 17,000 species of legumes (Zarucchi et al. 1993). This project is managed as a cooperative, involving approximately 20 research groups from five continents, and the information system is available internationally. Similar monographic projects have been established for several other plant groups (e.g., the Gymnosperm Database (www.conifers.org/) of taxonomic and specimen data for all 619 species of conifers in the world; the Brassicaceae species checklist and database, (www.cbif.gc.ca/pls/spec/brassicaceae) a species checklist of accepted names and synonyms for the Brassicaceae (Cruciferae) family; and Solanaceae Source (www.solanaceaesource.org), a database of names and descriptions of all taxa in the genus Solanum. There have also been floristic databases for regions, such as the Euro+Med PlantBase (www.emplantbase.org), which provides an on-line database and information system for the vascular plants of Europe and the Mediterranean region.
However, perhaps the most significant advance in recent years is The Plant List (www.theplantlist.org/), a collaboration between the Royal Botanic Gardens, Kew, in the UK; Missouri Botanical Gardens, St Louis, Missouri, in the USA; and other partners worldwide, to create the first working list of all known plants. It specifically addresses Target 1 of the Global Strategy for Plant Conservation for “a working list of known plant species” to be made available by 2010. It includes data on vascular plants (flowering plants, conifers, ferns and their allies) and of Bryophytes (mosses and liverworts). Currently it includes 1.25 million scientific plant names, of which 1.04 million are names of species rank. Of the species names included in The Plant List, about 300,000 (29%) are accepted names for species and about 480,000 (46%) are recorded as synonyms of those plant species. The status of the remaining 260,000 names is “unresolved” since the contributing data sets do not contain sufficient evidence to decide whether they should be accepted names or synonyms. The Plant List also includes a further 204,000 scientific plant names of infra-specific taxonomic rank linked to the species names.
Even more ambitious is the Catalogue of Life (www.catalogueoflife.org) project, developed by Species 2000 and the Integrated Taxonomic Information System (ITIS), which is planned to become a comprehensive catalogue of all known species of organisms on the planet. Rapid progress has been made recently in bringing together individual database projects, and the eleventh edition of the Annual Checklist has recently been published with 1,368,009 species. This is probably just slightly over two-thirds of the world's known species. The present Catalogue of Life is compiled from about 100 taxonomic databases from around the world. Many of these contain taxonomic data and opinions from extensive networks of specialists; the complete work contains contributions from more than 3000 specialists from throughout the taxonomic profession. The Species 2000 and ITIS teams peer review databases, select appropriate sectors and integrate the sectors into a single coherent catalogue with a single hierarchical classification. While the introduction of alternative taxonomic treatments and alternative classifications is planned, an important feature is that, for those users who wish to use it, a single preferred catalogue, based on peer reviews, will continue to be provided. It seems likely that in a few years we will take it for granted that such comprehensive lists of accepted names are available for our use, but the work involved in producing such lists should not be underestimated or go without being acknowledged.
Future challenges/needs/gaps
When the original text for this chapter was written, it would have been difficult to predict what challenges would be met and what gaps would remain, but clearly there have been advances—most notably in the use of molecular techniques to delimit and distinguish species and the production of a global list of accepted names. However, little if any progress has been made in moving beyond using traditional dichotomous keys as the routine basis for identification. Why? A cynic might point out that taxonomists are quite able to use traditional keys and have no real incentive to develop a more user-friendly means of identification, noting at the same time that both the use of molecular techniques and a global list of accepted names directly benefit their own taxonomic research. Yet there is no question that poor taxonomic training and lack of user-friendly identification aids are inhibiting the conservation and use of plant genetic resources. The gap remains, and the challenge will be to persuade funding agencies to address this gap. The most likely solution in the digital age is a mixture of text- and image-based interactive identification software that is taxa comprehensive and can run on cell phones or tablets that can easily be taken into the field. Allied to this, there remains a need for school and graduate training in applied taxonomy so that students and budding conservationists are not hobbled by deficiencies in their taxonomic skills.
Conclusion
Although there have been advances in identification science in recent years, poor taxonomic training and lack of user-friendly identification aids are currently inhibiting the conservation and use of plant genetic resources. This is resulting in (1) target species being missed in the field, (2) duplication of efforts in trying to locate priority taxa, (3) misidentification of conserved resources and (4) lack or poor utilization of conserved resources. Establishing time-bound targets and multi-institutional collaborative action has been shown to be very successful in generating the first working list of all known plant species. Could we learn from this example? Whatever approach is agreed upon, action is required now or our limited conservation resources will continue to be wasted. In the face of climate change and human mismanagement of the environment, can we continue to afford such waste?
Back to list of chapters on collecting
References and further reading
Al-Atawneh N, Shehadeh A, Amri A, Maxted N. 2009. Conservation Field Guide to Medics of the Mediterranean Basin. ICARDA, Aleppo, Syria.
Beentje H. 2010. The Kew Plant Glossary: An Illustrated Dictionary of Plant Terms. Royal Botanic Gardens, Kew, UK.
CBD. 2010. Global Strategy for Plant Conservation 2011-2020. CBD Secretariat, Ottawa, Canada (www.cbd.int/gspc/targets.shtml) .
CBOL Plant Working Group. 2009. A DNA barcode for land plants. Proceedings of the National Academy of Sciences 106(31):12794–12797.
FAO. 2010. Second Report on the State of the World’s Plant Genetic Resources for Food and Agriculture. FAO, Rome (www.fao.org/agriculture/seed/sow2/en/).
Feuillet C, Langridge P, Waugh R. 2008. Cereal breeding takes a walk on the wild side. Trends in Genetics 24:24–32.
Harris JG, Woolf Harris M. 2001. Plant Identification Terminology: An Illustrated Glossary. Spring Lake Publishing, Payson, Utah, USA.
Hickey M, King C. 2000. The Cambridge Illustrated Glossary of Botanical Terms. Cambridge University Press, Cambridge, UK.
House of Lords. 2002. What on Earth? The Threat to the Science Underpinning Conservation. Select Committee appointed to consider Science and Technology, House of Lords, London (www.publications.parliament.uk/pa/ld200102/ldselect/ldsctech/118/11802.htm).
Kress WJ, Wurdack KJ, Zimmer EA, Weigt LA, Janzen DH. 2005. Use of DNA barcodes to identify flowering plants. Proceedings of the National Academy of Sciences 102(23):8369–8374.
Lawrence A, Hawthorne W. 2006. Plant Identification: Creating User-Friendly Field Guides for Biodiversity Management. Earthscan, London.
Maxted N, Kell SP. 2009. Establishment of a Global Network for the In Situ Conservation of Crop Wild Relatives: Status and Needs. Background Study Paper No. 39. Commission on Genetic Resources for Food and Agriculture, FAO, Rome.
Maxted N, Mabuza-Dlamini P, Moss H, Padulosi S, Jarvis A, Guarino L. 2004. An ecogeographic survey: African Vigna. Systematic and Ecogeographic Studies of Crop Genepools 10. IPGRI, Rome.
Miller SE. 2007. DNA barcoding and the renaissance of taxonomy. Proceedings of the National Academy of Sciences 104(12):4775–4776.
Pankhurst RJ. 1991. Practical Taxonomic Computing. Cambridge University Press, Cambridge, UK.
Stevenson RD, Haber WA, Morris RA. 2003. Electronic field guides and user communities in the eco-informatics revolution. Conservation Ecology 7(1):3.
Zarucchi JL, Winfield PJ, Polhill RM, Hollis S, Bisby FA, Allkin R. 1993. The ILDIS Project on the world's legume species diversity. In: Bisby FA, Russell GF, Pankhurst RJ, editors. Designs for a Global Plant Species Information System. Oxford University Press, Oxford, UK. pp. 131–144.
Internet resources
Aids to Identification in Difficult Groups of Animals and Plants (AIDGAP): www.field-studies-council.org/publications/aidgaptesters.asp
Brassicaceae species checklist and database: www.cbif.gc.ca/pls/spec/brassicaceae
Catalogue of Life: www.catalogueoflife.org
eFloras: www.efloras.org/index.aspx
Euro+Med PlantBase: www.emplantbase.org
Gymnosperm Database: www.conifers.org/
International Legume Database and Information Service (ILDIS): http://ildis.org/
Linnaeus II: www.eti.uva.nl/products/linnaeus.php
Lucid: www.lucidcentral.com/
Solanaceae Source: www.solanaceaesource.org
The Plant List: www.theplantlist.org/
Chapter 17: Plant health and germplasm collectors
R. Macfarlane
Whitby Porirua, New Zealand
E-mail:bob.macfarlane(at)maf.govt.nz
G. V. H. Jackson
Queens Park Sydney, Australia
E-mail: gjackson(at)zip.com.au
E. A. Frison
Bioversity International, Rome, Italy
E-mail: e.frison(at)cgiar.org
|
2011 version |
1995 version |
||
|
Open the full chapter in PDF format by clicking on the icon above. |
|||
This chapter is a synthesis of new knowledge, procedures, best practices and references for collecting plant diversity since the publication of the 1995 volume Collecting Plant Genetic Diversity: Technical Guidelines, edited by Luigi Guarino, V. Ramanatha Rao and Robert Reid, and published by CAB International on behalf of the International Plant Genetic Resources Institute (IPGRI) (now Bioversity International), the Food and Agriculture Organization of the United Nations (FAO), the World Conservation Union (IUCN) and the United Nations Environment Programme (UNEP). The original text for Chapter 17: Plant Health and Germplasm Collectors, authored by E. A. Frison and G. V. H. Jackson has been made available online courtesy of CABI. The 2011 update of the Technical Guidelines, edited by L. Guarino, V. Ramanatha Rao and E. Goldberg, has been made available courtesy of Bioversity International.
Please send any comments on this chapter using the Comments feature at the bottom of this page. If you wish to contribute new content or references on the subject please do so here.
Back to list of chapters on collecting
Internet resources for this chapter
Abstract
When plant germplasm is moved nationally or internationally, there is a risk of the concomitant movement of pests (insects, pathogens, weeds and other organisms). Further, the quality of samples may be compromised by pests affecting viability during storage or, later, during multiplication and characterization.
This chapter suggests what collectors need to do to minimize these dangers. It is divided into (1) what to do when planning collecting missions (assembling the pest information, plant-health documents, intermediate quarantine, pest identification), (2) what to do in the field (minimizing the pest risk, record keeping, preservation of pest specimens), and finally, (3) what to do back at the base (preparing samples for inspection, phytosanitary treatments and certification, documents, and preparation of pests for identification). If followed systematically, these guidelines will facilitate plant germplasm reaching its destination unhindered, and will contribute to its value.
Introduction: the need for healthy germplasm
Uncontrolled movement of plant germplasm between countries spreads pests, but regulation of the movement of plant germplasm can help to reduce these risks. Pests, as defined by the International Plant Protection Convention (lPPC) (https://www.ippc.int/index.php?id=1110589&L=0), are “any species, strain or biotype of plant, animal or pathogenic agent injurious to plants or plant products” (IPPC 2010). (The IPPC facilitates cooperation between contracting parties to protect the world's cultivated and natural plant resources from the spread and introduction of pests of plants, while minimizing interference with the international movement of goods and people.)There are those that can be easily seen with the naked eye, such as insects, mites, slugs and snails, rats, plants and seeds, and those that are microscopic, such as fungi and the like, bacteria, phytoplasma, viruses and viroids. Previously, pests were considered to be only those organisms potentially damaging to crops, but now the term pest also includes newly introduced organisms that might damage ecosystems and plant and animal biodiversity.
Because there are dangers inherent in the movement of plant germplasm, most countries have legislation to regulate the entry (and sometimes the internal movement) of plants, plant parts and their products. In particular, the movement of wild collected germplasm causes significant concern, as its pest status is likely to be poorly known. Consignments of germplasm arriving in the importing country without proper documentation will be treated, reshipped or destroyed, irrespective of their botanical significance, the type of pest infestation or the status of the collector. To reduce the risk of accidental transfer of pests, germplasm should always be collected, processed and shipped in compliance with the phytosanitary requirements of the importing country. Contact details of most countries’ plant quarantine services can be found on the IPPC website.
It is possible, from long experience and sheer weight of knowledge of a plant species, that collectors might be better informed about the possible presence of quarantine pests associated with plant germplasm than the authorities in the importing country. In such cases, they should divulge this information, while at the same time ensuring that the germplasm collected and dispatched is free of pests to the extent that is possible.
There are other, perhaps less obvious, reasons why pests should be given attention when germplasm is collected. Pests might affect the quality, and therefore the usefulness, of germplasm samples. Infection by pathogens can reduce the viability of seeds during storage. When material is multiplied, growth may be distorted, colours altered and disease susceptibility increased. These changes may make it difficult, if not impossible, to collect characterization and preliminary evaluation data, and some important characteristics, crucial for plant-improvement schemes, might go undetected. In addition, infested samples are unlikely to be distributed. They cannot be grown out and regenerated and, if stored, they will remain unused and will deteriorate.
It is, therefore, important to know what pests are likely to be associated with the target gene pool. This will allow an assessment of the risks associated with moving the germplasm, as well as providing for appropriate measures to be devised to reduce the risk to a minimum. It is also important to document any pests present on the target species at the time of collecting. This information, part of the passport data of the sample, will improve the usefulness of the germplasm and will also help during quarantine examination.
For all these reasons, it is often useful to include a plant-protection specialist in collecting teams if funds and logistical considerations allow. Preferably, this should be a plant pathologist experienced in the species to be collected, as pathogens are more difficult than insects and mites to detect during collecting and to eradicate from plant samples. If a plant-protection specialist cannot participate in the collecting mission, collectors should become familiar with the major pests of the target species. In all cases, collectors will have to ensure that the phytosanitary requirements of the importing country have been met and proper documentation has been assembled so that plant samples reach their intended destination unhindered.
This chapter gives guidelines on how these issues may be addressed. It considers what must be done at the planning stage, while collecting in the field and, finally, just before samples are dispatched.
Planning the collecting mission
At the planning stage, attention must be given to the pests that might be encountered on the target species, and to the importing country’s regulations governing plant movement. The following questions need to be considered when assembling information on plant pests:
-
What pests have been recorded on the target species in the country of collecting, especially in the target area?
-
What plant parts are they found on?
-
How are the pests transmitted?
The following questions need to be answered to ensure compliance with phytosanitary regulations:
-
What is the final destination(s) of all samples, including subsamples?
-
What are the phytosanitary import requirements of the country(ies) of destination?
-
What are the procedures for obtaining a phytosanitary certificate in the country of collecting?
-
What are the procedures for verifying that the phytosanitary requirements of the importing country have been met prior to export?
Assembling information on pests
Since the first edition of this document was published, there has been an enormous change in the availability of information on plants and other organisms. The internet now allows immediate access to the most up-to-date information available in publications, research centres and museums worldwide. Perhaps the first point of contact for a collector seeking information on a plant or pest is to run a search for a pest under its presently accepted name (and at least one of its synonyms) through a search engine such as Google, Bing or Yahoo. This will generally turn up several significant leads for further enquiry, often with researchers familiar with the species being searched.
There are now literally hundreds of databases publicly available over the internet, and many others with restricted access for either commercial or confidentiality reasons. A selection of these is provided in the reference section, below, along with a selection of texts that may be consulted for information on the pests of specific crops. For example, the American Phytopathological Society publishes a particularly useful set of documents on the identification of crop plant diseases (www.cplbookshop.com/glossary/G583.htm).
On pest distributions, the following are important sources for accurate data on the worldwide distribution of plant pests and diseases of economic or quarantine importance:
|
Distribution maps of pests |
lIE (1968 et seqq.) |
|
|
Distribution maps of plant diseases |
IMI (1942 et seqq.) |
Collectors should confirm with the relevant institutes that these maps contain the most up-to-date information. Detailed descriptions, including notes on the transmission of many of the pests figured in the maps, can be sought from the following publications:
|
Descriptions of fungi and bacteria |
IMI (1964 et seqq.) |
|
|
Descriptions of plant-parasitic nematodes |
lIP (1972 et seqq.) |
|
|
Descriptions of plant viruses |
CMIIAAB (1970-1984) AAB (1985 et seqq.) |
The CABI Crop Protection Compendium (CPC) (www.cabi.org/cpc) is also a useful source of information on some 3000 pests, diseases, natural enemies and crops (with 400 recently commissioned sheets added in 2010) and basic information on 27,000 more species. The CPC helps with pest identification and distribution (including maps) and with phytosanitary and quarantine issues.
On viruses, CABI and the Australian National University have collaborated on a major database: the Virus Identification Data Exchange (VIDE). Viruses of Tropical Plants (Brunt et al. 1990) is an output of the database, as is Plant Viruses Online: Descriptions and Lists from the VIDE Database (Brunt et al. 1996). This site also provides links to some excellent web sites on plant viruses.
The Consultative Group on International Agriculture Research (CGIAR) supports a consortium of research centres around the world, many of which focus on specific crop plants:
-
Africa Rice Center
-
Bioversity International
-
International Center for Tropical Agriculture (CIAT)
-
Center for International Forestry Research (CIFOR)
-
International Maize and Wheat Improvement Center (CIMMYT)
-
International Potato Center (CIP)
-
International Center for Agricultural Research in the Dry Areas (ICARDA)
-
International Crops Research Institute for the Semi-Arid Tropics (ICRISAT)
-
International Institute of Tropical Agriculture (IITA)
-
International Rice Research Institute (IRRI)
-
World Agroforestry Centre (ICRAF)
Many of these research centres publish useful illustrated guides to the pests of their mandate crops. These are particularly useful in the field.
The CGIAR also has an online portal, the Crop Genebank Knowledge Base (http://cropgenebank.sgrp.cgiar.org/index.php?option=com_content&view=article&id=137&Itemid=238&lang=english), with guidelines for the safe transfer of germplasm for 15 seed crops and five clonally propagated crops under the mandate of CGIAR germplasm banks. In the introduction, the website states that it “summarizes information on current practices and guidelines for the safe transfer of germplasm gathered from the seed and crop health laboratories from CGIAR Centres in charge of the different crops”.
For each of the crops of interest there are sections on (1) germplasm import and export requirements, (2) technical guidelines for the detection and treatment of pests and pathogens and the safe transfer of germplasm and (3) best practices in place at the CGIAR Centres.
In addition, the Food and Agriculture Organization of the United Nations (FAO) and Bioversity International have published a series of booklets of crop-specific technical guidelines for the safe movement of germplasm (published under Bioversity’s previous names: International Board of Plant Genetic Resources and International Plant Genetic Resources Institute). They describe technical procedures that minimize the risk of introducing pests with the movement of germplasm for research, crop improvement, plant breeding, exploration or conservation. The recommendations in these guidelines are intended for germplasm for research, conservation and basic plant breeding programmes; they are not meant for trade and commercial consignments concerning the export and import of germplasm. Each booklet is divided into two parts: the first makes recommendations on how best to move the germplasm of the crop concerned and lists institutions recovering and/or maintaining healthy germplasm, the second covers the pests and diseases of quarantine concern, giving a description of therapy and indexing methodologies. So far, guidelines have been produced for the following crops (full citations and URLs are provided in the reference section below):
|
Acacia spp. |
Musa spp. |
There are many older texts that have crop-by-crop analysis of the problems and risks attendant on the transfer of plant germplasm, such as Hewitt and Chiarappa (1977), and these, too, can often contain information that remains useful.
Finally, pest surveys might also be consulted, if available, to determine which pests have been recorded on the target species in the collecting region. However, in many countries, such surveys are far from complete: sometimes, they have not been done at all, are outdated or do not cover the entire country, concentrating on the more easily accessible areas. Another problem is that pest surveys tend to record pests of crop plants, neglecting wild relatives, and rarely include native plants. This lack of information is a major barrier to the formulation of quarantine regulations appropriate to the exchange of germplasm of many plant species, including crop species.
Assembling the required plant health documents
It is essential to begin making phytosanitary arrangements early in the planning phase of any collecting expedition. Delays in obtaining the appropriate documents are common, but without these documents, the mission might have to be postponed or, worse, the samples destroyed. It is the responsibility of collectors to obtain the necessary documents in order to transfer plant germplasm. Two documents are commonly required for international plant transfer: an import permit and a phytosanitary certificate.
The import permit
The import permit must be obtained from the country or countries of destination of the germplasm well before the mission sets out. Information is also needed on how to obtain a phytosanitary certificate in the country of collecting and whether other authorizations are required to export germplasm, such as authorization under the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), which is an international agreement between governments that aims to ensure that international trade in specimens of wild animals and plants does not threaten the survival of these species. The FAO/IPPC Secretariat has a list of plant-protection services worldwide with contact addresses of the authorities responsible for issuing these documents. This list is available online at https://www.ippc.int/index.php?id=1110520&no_cache=1&type=contactpoints&L=0.
Regulations differ among countries according to the perceived risks involved in making the importation; the conditions of entry will be detailed on the import permit. Treatments may be required both in the country of export and in the country of import. If sub-samples are to be sent to several countries, permits must be obtained from each one. When no conditions apply and germplasm is allowed unconditional entry, it is advisable to obtain a document from the plant-protection service of the importing country to that effect. This will facilitate inspections at the border.
Usually, two copies of the import permit are provided. The top copy should accompany the consignment (if there is to be only one) and the other copy should be retained by the collector. A photocopy is usually allowed for multiple consignments.
It is recommended that collectors advise the importing phytosanitary border authorities of the number and approximate size of samples well ahead of arrival, so that the quarantine inspection service can plan to process the samples quickly. If the plants are to be grown in post-entry quarantine in the importing country or in a third country, then arrangements for this must be made during the planning phase of any expedition to ensure that facilities and space are available when needed.
The phytosanitary certificate
The phytosanitary certificate is issued by the quarantine authority of the exporting country, certifying that the product meets the phytosanitary regulations of the importing country. Consignments are inspected and the certificate issued if they are “free from quarantine pests and practically free of injurious pests” (see the IPPC model phytosanitary certificate, appendix 17.1 at the end of this chapter). A “quarantine pest” is different from a merely “injurious pest” in this statement in that it is of potential national economic importance to the country and not yet present there, or present but not widely distributed, and being actively controlled (IPPC 2010).
In some instances, in order to reduce the overall pest risk, germplasm consignments will need to be given phytosanitary treatments in the country of origin (see chapter 20 on the potential risks for seed viability of such treatments). Fumigation may be requested or the samples may be dipped or dusted in an insecticide or fungicide, given a hot-water treatment, or whatever is considered appropriate by the importing country. The treatments should be applied exactly as requested. If the collector is not confident that a treatment will be applied correctly in the exporting country and that it might fail to control the pest or that the germplasm might be damaged, it may be possible to negotiate with the importing country to have the treatment done under secure quarantine after arrival there. The permit may seek “additional declarations” verifying that these treatments have been applied as required. These, as well as details of the treatments, must be specified/declared on the phytosanitary certificate. Finally, the certificate should be signed by the authorized government representative.
Under no circumstances should alternative treatments to those specified on the import permit be applied without first requesting the authority of the importing country. Alternative treatments may be ignored by the importing quarantine inspector and a second treatment applied, which could reduce the viability of the germplasm. Likewise, if no treatments are requested, none should be given, since importing countries may wish to inspect or test germplasm consignments, and treatments already applied to seeds, for instance, may mask symptoms of seed-borne pathogens and interfere with laboratory tests. If seeds are treated prior to entry, contrary to the conditions of the permit, this could seriously jeopardize their importation.
Where germplasm samples are to be sent to more than one country, it is necessary to obtain phytosanitary certificates that comply with the requirements of each destination. It is important that the certificate(s) should be issued without amendment or erasure. Many countries refuse to accept altered certificates.
A fee may be charged for fumigation or disinfection treatments and, occasionally, for inspection.
Two copies of the phytosanitary certificate should be obtained, if possible. If there is only one, then the collector should make a copy. The original phytosanitary certificate should accompany the consignment and the copy should be kept with the other records of the collecting mission.
Documentation and intermediate quarantine
Collectors are responsible for arranging the documentation for germplasm samples that have to be grown in intermediate (third-country) quarantine. Such arrangements are necessary when it is unsafe to make transfers directly to the importing country. Procedures are essentially similar to those outlined above: an import permit must be obtained from the quarantine authority of the intermediate country. A copy of this should accompany the consignment, together with the phytosanitary certificate showing any treatments or endorsements requested on the permit. After the samples have been grown in intermediate quarantine and declared safe for further transfer, an import permit must be obtained from the country of final destination and a new phytosanitary certificate issued by the intermediate country.
Planning the identification of pests
Misidentifications of pests can seriously jeopardize the usefulness of consignments. Identification services for fungi, bacteria, nematodes are provided by CABI Global Plant Clinic (www.cabi.org/default.aspx?site=170&page=1017&pid=2301).
Costs vary depending on whether or not a country is a member of CABI. CABI also publishes useful directories of organizations, such as the International Mycological Directory (Hall and Hawksworth, 1990). It may be possible to arrange with the Danish Government Institute of Seed Pathology for Developing Countries (in Hellerup, Denmark) for the identification of important seed-borne diseases of tropical countries.
Identification of virus and virus-like infections is more problematical. Specimens need to be sent to institutes specializing in particular crop plants. Lists of institutes providing this service (such as the Tropical Virus Unit at the Institute of Arable Crops Research, Rothamsted Experimental Station, UK) can be found in the appropriate booklet in the FAO/IPGRI series of safe transfer guidelines. CABI also gives advice.
Identification of arthropods and many other biota can be obtained at a cost through the Natural History Museum, South Kensington, UK (www.nhm.ac.uk/about-us/contact-enquiries/identification-and-general-science-enquiries/index.html), and many other museums. In all cases, arrangements must be made well ahead of dispatch to allow the orderly processing of specimens. Import permits may be needed. If so, these must be obtained from the appropriate authorities in the country where specimens are to be examined. Collectors should ensure that the institutes making the identifications know where to send the results.
In the field
Minimizing the pest risk
Familiarity with the symptoms caused by pests and with which plant parts are most likely to be contaminated by the pests of concern is essential. In general, the risk of spreading pests with germplasm is greatest if rooted plants are moved. This is because of the likelihood that nematodes and other soil-borne pathogens will be present; these are difficult to treat without destroying the plant tissues. Other types of vegetative propagating material (e.g., stems, bulbs, corms, etc.) also present a risk, mainly because of infection from systemic pathogens. The international movement of seeds and pollen is considered safer, as fewer pests are harboured by these plant organs. Phytosanitary considerations may therefore contribute to the decision as to what plant part(s) to collect.
It may be possible to apply curative treatments to lessen or eradicate the pest risk. For surface-borne pathogens and insects, pesticide treatments and fumigation may be tried. Where virus, virus-like organisms and internally borne fungi and bacteria are a threat, thermotherapy and shoot-tip culture are most appropriate.
For vegetatively propagated species, transfer of germplasm as in vitro cultures will greatly reduce the pest risk. Nevertheless, it should be stressed that in vitro cultures do not eliminate the risk entirely. They should be complemented by indexing (testing) for viruses and virus-like organisms that are likely to be present in the area where the germplasm was collected.
The technical guidelines for the safe transfer of germplasm give general advice to collectors on the type of germplasm considered safe to move internationally, as well as detailed technical recommendations on how the germplasm may be treated to ensure that it is free of pests. In some instances, because of the severity of the pest and the difficulty of collecting healthy material from the field, the guidelines advise on transfer of material through a third country, where therapy and indexing procedures can be carried out to ensure freedom from internally borne pathogens. The general recommendations of the guidelines are useful even for crops not specifically covered in the series to date.
Recording data on pests
It is important for collectors to record the pests present on their target species and to note whether other pests are present in the target region. Noting that plants are free of pests in an area where pests are common is equally important. Collectors should attempt to describe the symptoms caused by pests. It is, however, often difficult for someone untrained in plant pathology or entomology to do this. Symptoms may be caused by a combination of several pests, or the causal agent may be obscured by the presence of a minor one or by an opportunistic saprophyte. Symptoms due to root attack or internal pathogens are often particularly difficult to interpret. Where there is doubt as to the identification of pests, plant specimens showing typical symptoms should be collected and dried or preserved by other means, as appropriate (see below).
A description of symptoms should include information on the following (Sonoda 1979):
-
the general condition of the plant
-
the plant part(s) affected
-
the type of damage
-
the stage of growth affected
Rating the severity of attack, both in terms of its effect on the individual plant(s) affected and in terms of the percentage of the population affected, will increase the value of the information. Descriptor lists are published by Bioversity International for many crops, cataloguing the important pests and giving scales of severity. These are available online at www.bioversityinternational.org/research/conservation/sharing_information/descriptor_lists.html.
Colour photographs showing the full range of symptoms, including close-ups of damaged areas and of the pests themselves, are often useful diagnostic tools (Sonoda 1979).
Farmers' knowledge of pests can be extensive and detailed. Some examples are given by Altieri (1993). Collectors can often complement the kinds of observations described above with discussions with knowledgeable local people.
Preservation of pests associated with germplasm samples
Correct identification of pests depends on the quality of the specimens prepared in the field. Collectors should be equipped at least with specimen bottles, alcohol (75% isopropyl alcohol) and formalin for preserving insects, mites and nematodes, and with newspapers and plant presses for making dried herbarium specimens of plants with fungal and bacterial diseases (chapter 27). Specimens may need to be shared among several institutes, and sufficient material should be collected to allow this.
Sonoda (1979) gives guidelines on capturing, killing and storing insects and other pests in the context of germplasm collecting. For insect pests, representative specimens of all life stages may be necessary for taxonomic identification. Insects can be captured using nets, by beating plants over a cloth or by using an aspirator. They can be killed using potassium cyanide or ethyl acetate, both of which are dangerous and should be clearly labelled and stored properly. Some insects must be pinned (e.g., beetles, flies and wasps); others can be stored in alcohol (e.g., caterpillars and other larvae, ants, aphids, scales and mealybugs) and others can be stored in small envelopes (e.g., moths and butterflies).
Dried specimens of diseased plants should include as much of the plant as possible, showing both old and new lesions. Fresh specimens can also be collected and stored in plastic bags. They will remain useful longer if refrigerated. Fungal and bacterial pathogens may be isolated from diseased plants in the field, but this requires sterile techniques and is not often feasible in the context of plant germplasm collecting.
Plants infected with viruses or virus-like organisms present the collector with the greatest challenge, as the material needs to be processed in different ways according to the type of pathogen. Where tissues are thought to contain non-cultivable mollicutes (formerly referred to as mycoplasma-like organisms), they need to be fixed in glutaraldehyde; whereas, tissues for virus examination may be sent fresh, dried as thin (1mm x 10mm) sections over calcium chloride (or silica gel) or as sap stained on electron microscope grids. Because of the complexity of the subject, it is essential that, prior to departure, collectors seek advice on the preservation of specimens from the institutes where the specimens are to be sent for examination.
Details of methods of preserving various kinds of diseased material can be found in The Plant Pathologist's Pocketbook (Waller et al. 2001). Methods for collecting and preserving different insect groups can be found in Bland and Jacques (1978), Borror et al. (1976) and British Museum (Natural History) (1974).
Back at base: treatment and dispatch of germplasm samples
This section gives a summary of the phytosanitary procedures involved in handling plant germplasm after it has been collected, along with brief notes on the dispatch of specimens for pest identification. For other aspects of the tasks that will need to be undertaken once back at base, see Chapter 28.
Inspection
Missions should carefully prepare germplasm samples before they are presented to quarantine authorities for inspection, treatment and certification.
-
Germplasm samples should be carefully inspected for pests, insects and mites as well as for lesions or colour patterns that might denote fungal, bacterial or viral pathogens. Where such pests, or symptoms of pests, are present, the pests and/or the symptom-bearing seeds should be removed.
-
Bare-rooted plants should be thoroughly washed to ensure they are free of soil, which might harbour nematodes and other soil-borne pathogens.
-
Seeds and pollen should be free of debris. If debris is present, it should be removed.
Phytosanitary treatments and certification
-
If mandatory treatments are prescribed on the import permit or endorsements are required, these should be given by the relevant government authority exactly as requested.
-
After treatments have been applied, they should be detailed on the phytosanitary certificate, together with any other endorsements requested by the importing country.
-
The phytosanitary certificate should bear the stamp of the organization issuing the certificate and should be signed by an authorized officer. Many countries are now issuing phytosanitary certificates electronically with stamps and signatures added in the computer (known as ePhytos). These are equally valid and may ease on-arrival arrangements because they are increasingly sent to the importing country digitally soon after they are issued.
-
Collectors should ensure that the phytosanitary certificate contains the following information:
-
name and address of the exporter
-
name and address of the consignee
-
number of samples of each species in the consignment
-
botanical name of each species
-
details of any phytosanitary treatments applied
-
additional endorsements required by the import permit
-
Documents accompanying germplasm consignments
-
The original phytosanitary certificate, plus a copy of the import permit, should accompany each consignment. Most importing countries will allow photocopies of import permits if there are multiple shipments, but it would be best to confirm this from the quarantine authorities of the importing country if there are any doubts. A copy of the import permit should be placed on the outside of the package so it can be forwarded to the plant quarantine authorities without the need to open it. A photocopy of the permit should be included inside the package in case of damage to the outside copy. However, this may vary from country to country. For example, regulations in the United States specify that all documents should be inside the package.
-
A copy of all documents sent with the consignments should be retained by the collector.
Preparation of samples for pest identification
Arrangements should be made in advance of the fieldwork with the institutes that are to receive samples for pest identification. Permits may have to be obtained to comply with the quarantine requirements of the country where samples are to be sent. Some additional points:
-
All material sent for identification purposes, whether preserved insects and mites, dried plant voucher specimens of diseased plants or living plant material for diagnosis of internal pathogens, should be labelled
-
with a reference number, as well as
-
the botanical name of the host plant
-
the locality where collected
-
the date of collecting
-
the name of the collector(s)
-
-
Collectors should keep a copy of the information accompanying each specimen.
-
Samples of seeds and pollen may have to be sent for viability testing, as well as for inspection for internally-borne pathogens, and weeds. Samples should be properly dried before dispatch.
-
Collectors should include the name of the person (and address) to whom the identification(s) should be sent.
Future challenges/needs/gaps
And what of the future? The challenge is for samples to move ever faster, and ever more safely, between countries, from places of collection to places of storage and other use. This requires collectors and quarantine officials to work together in more consistent and coherent ways – in itself a major challenge.
Collectors and the users of plant germplasm would like quarantine officials to act faster. Quarantine officials (ever mindful of their mandate to protect crops and indigenous flora from new pest incursions) need time if they are to do their job thoroughly. Tensions can arise from these, seemingly, different ways of viewing germplasm, but this is a false dichotomy. All sides have to work together if satisfactory outcomes are to be achieved: the rapid international movement of germplasm safe from risk.
If this is not achieved, there will be – or continue to be – adverse consequences. We know from anecdotal evidence that long delays in quarantine bring frustrations and, unfortunately, a temptation among some to short-circuit official systems, especially where these are not well developed. It is the responsibility of those sponsoring collecting missions to forbid such practices.
So are there ways to reduce the time it takes to process samples, check for associated pests? Some suggestions are obvious: crop-specific guidelines for testing germplasm for the pests of concern need to be produced, and they need to be updated constantly. Those produced by Bioversity International (also under its previous names as IBPGR and IPGRI) are expensive to produce and become outdated too soon. This needs to change. Documents need to be posted on the internet, and frequent revisions made as new data comes to hand. The linking of institutes – international and national – dealing with indexing technologies of specific crops needs to continue. And conformity of national quarantine regulations needs to be achieved. There are still countries that re-index samples, no matter that they have been indexed elsewhere, causing considerable frustration to would-be users.
Another question is whether molecular technologies can assist in speeding up the processing of germplasm samples. The most likely answer is both a “yes” and a “no”. Several current programmes that “fingerprint” and “bar-code” plants and animals will, undoubtedly, lead to speedy identifications of pests attached to germplasm being moved internationally. It may also speed up the process of determining whether pathogens are present within plant tissues.
The problem, however, is what to do about the detection of DNA within a plant that differs from that of the plant itself, particularly if there are no symptoms of any kind. Currently, plants that are symptomless after months or years (most often a crop cycle) in quarantine are released to the importer. In future, national phytosanitary officials may have to deal with the quandary of what to do about symptomless plants containing apparently alien DNA. There is no guarantee that once released into a new environment the endophytic organism will not be transferred to another plant (perhaps by an insect vector) and become a pest. Decision making in this area is set to become extremely difficult.
As mentioned already in this chapter, the internet has grown greatly over the years since this book was first published, and is now of immense value to researchers and national phytosanitary officials alike. They now have almost instant access to all the knowledge available on a particular organism, much of it being kept up to date. Perhaps the next phase, already beginning in some countries, will be to enable researchers, industry and governments to collaborate, use expertise, share data and information, and generate intelligence through the development of information technologies akin to the popular social networks. An example of this is the web portal of the Australian Biosecurity Intelligence Network (ABIN): www.abin.org.au/web/index.html.
Development of biosecurity networks will be a challenge within countries where researchers may guard certain information prior to publication, but it will be even more challenging between countries where the trade implications of the presence or absence of a pest can have huge economic consequences. Nevertheless, the benefits of information sharing are already self-evident following the growth of the internet, and it is to be hoped that attempts at greater information sharing through networking will be equally positive.
Conclusions
Countries throughout the world are keen to safeguard agriculture, forestry and the environment from potentially threatening invasive pest species. This commitment is regulated under the International Plant Protection Convention with support, in recent years, from the World Trade Organization’s Agreement on the Application of Sanitary and Phytosanitary Measures (the SPS Agreement). Governments apply measures for food safety and animal and plant health – sanitary and phytosanitary measures – to impede the spread of pests. Collectors of germplasm must be aware of these developments, and the potential harm that the unrestricted movement of germplasm could cause. They must ensure that samples conform to standards set under both national legislation and international regulations. Failure to do this could jeopardize collecting missions.
Back to list of chapters on collecting
References and further reading
General
AAB. 1985 et seq. Descriptions of Plant Viruses. Association of Applied Biologists, Wellesbourne, UK.
Altieri MA. 1993. Ethnoscience and biodiversity: key elements in the design of sustainable pest management systems for small farmers in developing countries. Agriculture, Ecosystems and Environment 46:257–272.
Bland RG, Jacques HE. 1978. How To Know Insects. WC Brown Company Publishers, Dubuque, Iowa.
Borror DJ, Delong DM, Triplehorn CA. 1976. An Introduction to the Study of Insects. Holt, Rinehart and Winston, New York.
British Museum (Natural History). 1974. Insects. Instructions for collectors No.4a. British Museum (Natural History), London.
Brunt AA, Crabtree K, Gibbs A. 1990. Viruses of Tropical Plants. CAB International, Slough, UK.
Brunt AA, Crabtree K, Dallwitz M, Gibbs A, Watson L, Zurcher E, editors. 1996. Plant Viruses Online: Descriptions and Lists from the VIDE Database. CAB International, Wallingford, UK. Available online (accessed 15 August 2011): www.agls.uidaho.edu/ebi/vdie/refs.htm.
CMI/AAB. 1970-1984. Descriptions of Plant Viruses. Sets 1–18. CAB International and Association of Applied Biologists, Slough and Wellesbourne, UK.
FAO. 1993. Directory of Regional Plant Protection Organizations and National Plant Quarantine Services. AGPP/Misc /93/1. FAO, Rome.
FAO. 2011. International Standards for Phytosanitary Certificates. ISPM 12. FAO, Rome. Available online (accessed 15 August 2011): https://www.ippc.int/file_uploaded/1307528241_ISPM_12_2011_En_2011-05-03(Corre.pdf.
Hall GS, Hawksworth DL. 1990. International Mycological Directory. CAB International, Wallingford, UK.
Hewitt WB, Chiarappa L. 1977. Plant Health and Quarantine in International Transfer of Genetic Resources. CRC, Cleveland, Ohio.
IIE. 1968 et seq. Distribution Maps of Pests. Series A (Agriculture). International Institute of Entomology (formerly Commonwealth Institute of Entomology) and CAB International, Wallingford, UK.
IIP. 1972 et seq. IIP Descriptions of Plant-Parasitic Nematodes. International Institute of Parasitology (formerly Commonwealth Institute of Helminthology) and CAB International, Wallingford, UK.
IMI. 1942 et seq. IMI Distribution Maps of Plant Diseases. International Mycological Institute (formerly Commonwealth Mycological Institute) and CAB International, Wallingford, UK.
IMI. 1964 et seq. IMl Descriptions of Fungi and Bacteria. International Mycological Institute (formerly Commonwealth Mycological Institute) and CAB International, Wallingford, UK.
IPPC. 2010. Glossary of Phytosanitary Terms. ISPM No. 5. International Plant Protection Convention, Rome. Available online (accessed 15 August 2011): https://www.ippc.int/file_uploaded/1273490046_ISPM_05_2010_E.pdf.
Johnston A, Booth C. 1983. Plant Pathologist's Pocketbook. CAB International, Wallingford, UK.
Nelson SC, Bushe BC. 2006. Collecting Plant Disease and Insect Pest Samples for Problem Diagnosis. SCM-14. Cooperative Extension Service, University of Hawaii at Manoa, Honolulu, Hawaii. Available online (accessed 29 August 2011): www.ctahr.hawaii.edu/oc/freepubs/pdf/SCM-14.pdf.
Sonoda RM. 1979. Collection and preservation of insects and pathogenic organisms. In: Mott GO, Jimenez A, editors. Handbook for the Collection, Preservation and Characterization of Tropical Forage Germplasm Resources. CIAT, Cali, Colombia.
Waller JM, Lenné JM, Waller S. 2001. Plant Pathologists’ Pocketbook. 3rd Edition. CAB International, Wallingford, UK.
American Phytopathological Society online bookshop: www.cplbookshop.com/glossary/G583.htm
Australian Biosecurity Intelligence Network (ABIN): www.abin.org.au/web/index.html
Bioversity International descriptor lists: www.bioversityinternational.org/research/conservation/sharing_information/descriptor_lists.html
CABI Crop Protection Compendium (CPC): www.cabi.org/cpc
Crop Genebank Knowledge Base: http://cropgenebank.sgrp.cgiar.orgindex.php?option=com_content&view=article&id=137&Itemid=238&lang=english
Descriptions of fungi and bacteria (IMI): www.cabi.org/default.aspx?site=170&page=1016&pid=2214
Descriptions of plant viruses (CMIIAAB): www.dpvweb.net
Distribution maps of pests (lIE): www.cabi.org/default.aspx?site=170&page=1016&pid=2212
Distribution maps of plant diseases (IMI): www.cabi.org/default.aspx?site=170&page=1016&pid=2210
FAO/IPPC list of plant-protection services worldwide: https://www.ippc.int/index.php?id=1110520&no_cache=1&type=contactpoints&L=0
Global Plant Clinic: www.cabi.org/default.aspx?site=170&page=1017&pid=2301
International Plant Protection Convention (lPPC): https://www.ippc.int/index.php?id=1110589&L=0
Natural History Museum, South Kensington, UK: www.nhm.ac.uk/about-us/contact-enquiries/identification-and-general-science-enquiries/index.html
Examples of useful databases on plants and pests
|
Arthropods of Economic Importance: Agromyzidae |
|
|
Arthropods of Economic Importance: Diaspididae |
http://nlbif.eti.uva.nl/bis/diaspididae.php?menuentry=inleiding |
|
Australian Faunal Director |
www.environment.gov.au/biodiversity/abrs/online-resources/fauna/afd/home |
|
Databases and Publications from Kew |
www.kew.org/science-research-data/databases-publications/index.htm |
|
Descriptions of Plant Viruses |
|
|
Diseases and disorders of cultivated palms |
|
|
EcoPort |
|
|
EPPO Database on Diagnostic Expertise |
|
|
Fauna Europea |
|
|
Flora of New Zealand |
|
|
ICTVdB Index of Viruses |
|
|
Lucidcentral Key Search |
www.lucidcentral.com/Keys173/SearchforaKey/tabid/217/language/en-US/Default.aspx |
|
Index Fungorum |
|
|
Mansfeld's World Database of Agricultural and Horticultural Crops |
http://mansfeld.ipk-gatersleben.de/pls/htmldb_pgrc/f?p=185:3:4166990628405234 |
|
NLBIF Biodiversity Data Portal |
|
|
Landcare Research (New Zealand) |
|
|
Pacific Islands Pest List Database |
|
|
Plant Viruses Online |
|
|
The Global Lepidoptera Names Index |
www.nhm.ac.uk/research-curation/research/projects/lepindex/index.html |
|
USDA ARS Fungal Databases |
|
|
USDA ARS Germplasm Resources Information Network (GRIN) Taxonomy for Plants |
|
|
USDA ARS Systematic Entomology Laboratory |
|
|
Wikispecies |
FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm Series
Acacia spp.
Old KM, Vercoe TK, Floyd RB, Wingfield MJ, Roux J, Neser S, editors. 2002. Acacia spp. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm No. 20. FAO/IPGRI, Rome.
Allium spp.
Diekmann M. 1997. FAO/IPGRI Technical Guidelines for the Safe Movement of Allium spp. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm No. 18. FAO/IPGRI, Rome.
Cacao
Frison EA, Diekmann M, Nowell D, editors. 2000. FAO/IPGRI Technical Guidelines for the Safe Movement of Cocoa Germplasm. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm No. 20. FAO/IPGRI, Rome.
Cassava
Frison EA, Feliu E, editors. 1991. FAO/IBPGR Technical Guidelines for the Safe Movement of Cassava Germplasm. FAO/IBPGR, Rome.
Citrus
Frison EA, Taber M, editors. 1991. FAO/IBPGR Technical Guidelines for the Safe Movement of Citrus Germplasm. FAO/IBPGR, Rome.
Coconut
Frison EA, Putter CAJ, Diekmann M, editors. 1993. FAO/IBPGR Technical Guidelines for the Safe Movement of Coconut Germplasm. FAO/IBPGR, Rome.
Edible aroid
Zettler FW, Jackson GVH, Frison EA. 1989 FAO/IBPGR Technical Guidelines for the Safe Movement of Edible Aroid Germplasm. FAO/IBPGR, Rome.
Eucalyptus spp
Ciesla WM, Diekmann M, Putter CAJ, editors. 1996. Eucalyptus spp. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm No. 17. FAO/IPGRI, ACIAR & ASEAN, Rome.
Grapevine
Frison EA, lkin R, editors. 1991. FAO/IBPGR Technical Guidelines for the Safe Movement of Grapevine Germplasm. FAO/IBPGR, Rome.
Legumes
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD, editors. 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. FAO/IBPGR, Rome.
Musa spp.
Diekmann M, Putter CAJ. 1996. FAO/IPGRI Technical Guidelines for the Safe Movement of Musa Germplasm. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm No. 15. 2nd Edition. FAO/IPGRI, Rome.
Pinus spp.
Diekmann M, Sutherland JR, Nowell DC, Morales FJ, Allard G, editors. 2002. Pinus spp. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm No. 21. FAO/IPGRI, Rome.
Potato (Solanum tuberosum)
Jeffries C J. 1998. Potato. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm No. 19. FAO/IPGRI, Rome.
Small fruits
Diekmann M, Frison EA, Putter CAJ, editors. 1994. FAO/IPGRI Technical Guidelines for the Safe Movement of Small Fruit Germplasm. FAO/IPGRI, Rome.
Small grain temperate cereals
Diekmann M, Putter CAJ, editors. 1995. Small Grain Temperate Cereals. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm No. 14. FAO/IPGRI, Rome.
Stone fruits
Diekmann M, Putter CAJ, editors. 1994. FAO/IPGRI Technical Guidelines for the Safe Movement of Stone Fruit Germplasm. FAO / IPGRI Technical Guidelines for the Safe Movement of Germplasm No. 16. FAO/IPGRI, Rome.
Sugarcane
Frison EA, Putter CAJ, editors. 1993. FAO/IBPGR Technical Guidelines for the Safe Movement of Sugarcane Germplasm. FAO/IBPGR, Rome.
Sweet potato (Ipomoea batatas)
Moyer JW, Jackson GVH, Frison EA. 1989. FAO/IBPGR Technical Guidelines for the Safe Movement of Sweet Potato Germplasm. FAO/IBPGR, Rome.
Vanilla
Pearson MN, Jackson GVH, Zettler FW, Frison EA. 1991. FAO/IBPGR Technical Guidelines for the Safe Movement of Vanilla Germplasm. FAO/IBPGR, Rome.
Yam (Dioscorea spp.)
Brunt AA, Jackson GVH, Frison EA. 1989. FAO/IBPGR Technical Guidelines for the Safe Movement of Yam Germplasm. FAO/IBPGR, Rome.
The American Phytopathological Society (APS) publications on the identification of plant diseases (www.apsnet.org/publications/Pages/default.aspx)
|
Compendium of Corn Diseases (Third Edition) |
Shade Tree Wilt Diseases |
Appendix 17.1: IPPC Model Phytosanitary Certificate
(Download IPPC Model Phytosanitary Certificate (0.9 MB) for printing purposes)
 |
|
Source: FAO (2011) |
More Articles...
- Chapter 25: Collecting pollen for genetic resources conservation
- Chapter 20: Collecting and handling seeds in the field
- Chapter 13: Published information resources for plant germplasm collectors
- Chapter 10: Published sources of information on wild plant species
- Chapter 8: Sources of information on existing germplasm collections
- Chapter 19: Collecting and recording data in the field: media for data recording
- Chapter 22: Collecting vegetative material of forage grasses and legumes
- Chapter 24: Collecting in vitro for genetic resources conservation
- Chapter 15/16: Mapping the ecogeographic distribution of biodiversity and GIS tools for plant germplasm collectors
- Chapter 27: Collecting herbarium vouchers



 Collecting
Collecting

.jpg)
