Viruses - faba bean
Contributors to this section: ICARDA, Syria (Siham Asaad, Abdulrahman Moukahal).
Scientific name
Bean Yellow Mosaic Virus(BYMV)
Other scientific names
Bean virus 2, Canna mosaic virus ,Gladiolus mosaic virus, Gloriosa stripe mosaic virus.
Significance
During pulse surveys from 2000-2005, BYMV infections were uncommon and found in less than 1% of surveyed crops. However, BYMV was sometimes found in faba bean crops and occasionally in peas with within crop virus incidences of 1-15% and up to 7% respectively. In NSW, a small survey showed that faba bean crops had an average within crop incidence of BYMV of 26% of plants (ranging from 1-63%) (van Leur et al. 2002). Field surveys in 1998-1999 showed that some faba bean and field pea crops were infected with BYMV and the within crop virus incidences were 1-31% and 1-11% respectively. Plot trials showed that lupins infected with the necrotic strain of BYMV can have grain yields reduced by 95%.
Symptoms
Leaves of infected plants show mild mosaic followed by narrowing. The new growth from leaf axils has leaves that are narrow, elongated and light green. Early infections adversely affect plant growth and yield.
Lentils develop mild mosaic, light green or yellow leaves, reduction in leaf size and stunting may occur. Infected plants produce very little seed.
Narrow-leaf lupins, infected with the necrotic strain of BYMV initially develop yellow leaves followed by necrosis of growth tips and plant death. Non-necrotic strains of BYMV cause yellowing and dwarfing but do not cause death of the plant.
Subterranean clover plants develop leaf mottling, leaf deformation and distinct yellowing between the veins. Plants become dwarfed and symptoms usually occur in patches and along the edges of paddocks.
Hosts
The host range of BYMV is wide and not limited to Fabaceae. The virus is reported to infect nearly 200 species in 14 families. Temperate pulse hosts include chickpeas, faba beans, field peas, lentils and lupins. Temperate legume pasture hosts include lathyrus, lucerne, vetch and medic and clover species. BYMV has a number of sub-tropical and tropical pulse hosts, including soybeans, peanuts and French beans as well as legume pasture hosts. It also infects ornamental hosts, the most common being gladiolus species.
Geographic distribution
Belarus, Belgium: Bulgaria, Czech Republic,Denmark, Finland, Former Yugoslavia, France, Germany, Greece, Hungary, Lithuania, Netherlands, Poland, Portugal, Romania, Russian Federation, Russia (Europe),Russian Far East, Spain, Sweden, Ukraine, United Kingdom, China, Georgia (Republic), India , Indonesia, Iran, Israel, Japan, Jordan, Kazakhstan, Korea, Lebanon, Pakistan, Syria, Turkey, Uzbekistan, Egypt, Kenya, Libya, Morocco, South Africa, Sudan, Tanzania, Tunisia Zambia, Zimbabwe, Western Hemisphere, Canada, Chile, Dominican Republic, Jamaica, Mexico, Montserrat, Peru, USA, Alabama, Kentucky, Tennessee, Australia, New Zealand.
Biology and transmission
BYMV is transmitted by more than 50 aphid species in a non-persistent manner: the main species are Acyrthosiphon pisum, Aphis fabae, A. gossypii, Aulacorthum solani Brevicoryne brassicae, Myzus persicae and Rhopalosiphum maidis. During surveys in Victoria the following BYMV vectors in pulse crops were found : Acyrthosiphon kondoi, Aphis craccivora, Aulacorthum solani, Brevicoryne brassicae and Myzus persicae. Acyrthosiphon kondoi, Aphis craccivora, Myzus persicae, Brachycaudus rumexicolens, Lipaphis erysimi, Rhopalosiphum maidis, R. padi, Sitodion miscanthi and Therioaphis trifolii have been reported as BYMV vectors.
The virus is also transmitted through seed of most temperate pulses, including faba beans, field peas, lentils, and lupins and through seed of a number of forage legumes and clovers. In Victoria, 18% of lentil seed lots tested had BYMV infections of 0.1-0.9%. In WA, the following BYMV seed transmission is reported: yellow and white lupins 3-6%, field peas 0.3-0.8%, faba beans 0.4%, lathyrus 0.1-0.2% and vetch 0.5%.
Seed transmission of BYMV in medics and clovers has also been reported in WA as follows: Melilotus indica (0.5%), Medicago polymorpha (0.9%), M. truncatula (0.3%), M. indica (1%), Trifolium arvense (0.1%), T. campestre (0.2%) and T. glomeratum (0.05%).
Detection/indexing method at ICARDA
- TBIA (Tissue Blot Immuno Assay test), Enzyme-linked immunosorbent assay (ELISA)
Treatment/control
- Seed is considered to be one of the main sources of BYMV, therefore sowing virus tested seed is recommended and commercial seed tests are available.
- BYMV causes heavy losses in narrow leafed lupins, so only virus tested seed is recommended for sowing. Virus resistant lupin varieties are now available.
- BYMV-infected pastures are another major source of the virus, which is then spread to crops by aphids. Chemical control of aphids is not an effective method for controlling non-persistently transmitted viruses such as BYMV.
- Pulse crops should be sown away from legume pastures to minimise the spread of BYMV.
- The spread of virus can also be reduced by controlling weed hosts from in and around paddocks.
Procedure followed at the centers in case of positive test
- Destroying seed, production healthy seed
References and further reading
Temperate Pulse Viruses: Bean Yellow Mosaic Virus (BYMV)
http://www.staff.kvl.dk/~thluj3/symptoms/fababymv.html
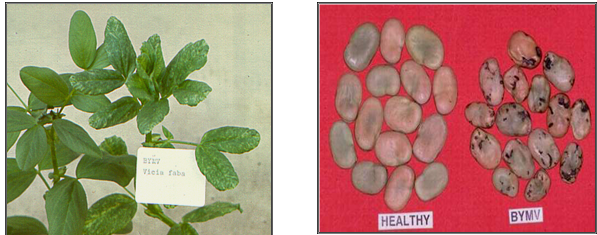 BYMV (photos:www.staff.kvl.dk and www.dpi.vic.gov.au) |
Scientific name
Broad Bean Stain Virus (BBSV).Lloyd, Smith & Jones, 1965.
Other scientific names
Broad bean F1 virus, Broad bean Evesham stain virus
Significance
Nineteen genotypes of lentil were studied in Syria for variability in seed transmission rates and yield losses induced by broad bean stain virus (BBSV) infection. Seed transmission rates of BBSV varied between 0.2% and 32.4%, whereas yield loss due to infection varied between 14% and 61%.
Symptoms
The disease is characterized by a mild mottling on the leaves.
The affected plants are reduced in growth, which is easily recognized, especially when they are compared with healthy plants. Yield reduction of 15-16% could occur in some different cultivars. Seed from infected plants occasionally show dark staining on the seed.
Hosts
Hosts restricted to Leguminosae; inoculated 44 species in 19 dicotyledonous families, but the virus infected only 7 species of legume: Crotalaria spectabilis, Lupinus hirsutus, Melilotus alba, Phaseolus vulgaris (French bean), Pisum sativum (pea), Trifolium incarnatum (crimson clover) and Vicia faba (broad bean).
Geographic distribution
Europe, Austria, Hungary, Italy, Poland, United Kingdom, Asia (as a whole), China, Syria, Turkey, Africa (as a whole) Egypt.
Biology and transmission
Transmission by Vectors: Transmitted by a vector; an insect; Apion vorax, Sitona spp.; Coleoptera.
Transmission through Seed: Common in certain varieties of broad bean, transmitted to up to 10% of the progeny of infected plants.
Transmission by Dodder: Not tested.
Virus transmitted by mechanical inoculation; transmitted by grafting; transmitted by seed (up to 10% in some Vicia faba cultivars).
Detection/indexing method at ICARDA
- Tissue Blot Immuno Assay test (TBIA), Enzyme-linked immunosorbent assay (ELISA).
Treatment/control
- For the three seed-borne viruses (BBSV, PSbMV and CMV) it is important to avoid the use of virus-infected seed lots.
- Rogue early infected plants to prevent further disease spread.
- For the aphid-transmitted viruses, spray systemic aphidicides, especially in lentil nurseries or seed multiplication plots. This should be considered in situations where winged forms of aphids begin to appear. Spraying will help suppress aphid populations in lentil fields.
- For BBSV, which is transmitted by weevils, treat seed with promet (12 ml/kg seed) to help reduce the vector and hence disease spread.
- All these viruses have a wide host range including other legumes such as dry peas, phaseolus bean, faba bean, alfalfa and clover.
Procedure in case of positive test
- Destroying infected seed, producing healthy seed
References and further reading
http://www.dpvweb.net/dpv/showdpv.php?dpvno=029
http://image.fs.uidaho.edu/vide/descr112.htm#Taxonomy
http://www.icarda.org/Publications/Field_Guides/Lentil/Lent7.Html
http://www.cababstractsplus.org/abstracts/Abstract.aspx?AcNo=19916777532
http://www.padil.gov.au/pbt/index.php?q=node/20&pbtID=152
 BBSV (photos:www.padil.gov.au) |
Scientific name
Pea Seed-borne Mosaic Virus (PSbMV)
Other scientific names
Pea fizzle top virus, Pea leaf rolling virus, Pea leaf roll mosaic virus, Pea leaf rolling mosaic virus.
Significance
PSBMV is of economic importance in pea, faba bean and lathyrus, mainly due to its effect on seed quality. It has been mistakenly considered to be a minor disease because it often causes only minor yield loss and mild symptoms, However, at the International Centre for Agriculture in the Dry Areas (ICARDA), Syria, glasshouse studies on yield losses due to PSBMV in chickpea, faba bean, lentil and pea showed losses of 66%, 40.5%, 44.6% and 49.2% respectively. Surveys of pulse crops over a number of years in Vic and SA showed that up to 15% of crops were infected with PSBMV, with a within crop incidence of up to 5% of plants. In surveys in WA in 1999, PSBMV was found in 42% of field pea crops and the level of virus detected in pea seed stocks was up to 63%. The surveys indicated that PSBMV has a severe effect on seed quality of pulse crops with seed coat symptoms evident on >80% of faba bean, >50% of lathyrus species and field peas and 5% of chickpea seed.
Symptoms
The disease is characterized by a mild mottling on the leaves, which is not easily recognizable because of the small size of the lentil leaf.
The affected plants are reduced in growth, which is easily recognized, especially when they are compared with healthy plants. Yield reductions of 15-16% could occur in some different cultivars. Seeds from infected plants occasionally show dark staining on the seed.
Lentils may show no symptoms or there may be chlorosis in new shoots, mottling on leaves, shoot tip necrosis and stunting of plants.
Hosts
The natural host range of PSBMV is limited to the Fabaceae. It infects temperate pulses (chickpea, faba bean, field pea, lentil) other legumes (garden pea, narbon bean) and pastures (lathyrus and vetch). A number of PSBMV pathotypes have been recognised by their ability to infect a number of pea differential genotypes.
Geographic distribution
Belgium, Bulgaria, Czechoslovakia (former -), Denmark, Finland, France, Germany, Netherlands, Poland, Romania, Russia (Europe), Sweden, Switzerland, United Kingdom, China, Taiwan, India, Israel, Japan, Jordan, Lebanon, Nepal, Syria, Turkey, Egypt, Ethiopia, Libya, Morocco, South Africa, Sudan, Tanzania, Tunisia, Zambia, Zimbabwe, Western Hemisphere, Brazil, Canada, Peru, USA, Washington Australia , New Zealand.
Biology and transmission
The virus is believed to have spread worldwide through the exchange of infected seed. Seed transmission rates of up to 100% in peas and up to 44% in lentils have been reported. In Victoria, we have detected PSBMV at low levels in some commercial chickpea seedlots (0.4% of seed) and at higher levels in field pea and lentil seedlots (greater than 2% of seed). In the USA, 3% PSBMV infection in pea seedlots and 32-40% in lentil seedlots have been reported. At ICARDA, PSBMV was found to be transmitted through lentil seeds at rates of up to 44%. PSBMV is also transmitted in a non-persistent manner by more than 20 aphid species and by mechanical means. The most efficient vector is the pea aphid (Acyrthosiphon pisum). The other species are as follows: A. pelargoni, A. sesbaniae, Aphis craccivora, A. fabae, A. gossypii, A. nasturtii, Aulacorthum circumflexum, A. solani, Brevicoryne brassicae, Cryptomyzus ribis, Dactynotus escalantii, Macrosiphum avenae, M. euphorbiae, M. pisi, M. rosae, Metopolophium dirhodum, Myzus persicae, Ovatus crataegarius, Phorodon cannabis, Rhopalosiphum padi and Semiaphis dauci. In Victorian pulse crop surveys, we have found cowpea aphid (Aphis craccivora), foxglove aphid (Aulacorthum solani), and green peach aphid (Myzus persicae), which are all vectors of PSBMV.
Detection/indexing method in place at the CGIAR Center at ICARDA
- (TBIA) Tissue Blot ImmunoAssay test, Enzyme-linked immunosorbent assay (ELISA)
Treatment/control
- Seed is considered to be the main source of PSBMV, therefore sowing virus tested seed is the most effective way of controlling this virus.
- Chemical control of aphids is not an effective method for controlling non-persistently transmitted viruses, such as PSBMV.
- Pulse crops should be sown away from other legumes to minimise the spread of PSBMV.
Procedure followed at the centers in case of positive test
- Destroy seed, produce healthy seed
Referencs and further reading
Temperate Pulse Viruses: Pea Seedborne Mosaic Virus (PSBMV)
http://www.agls.uidaho.edu/ebi/vdie//vide/descr575.htm
 PSbMV (photos:www.dpi.vic.gov.au) |
Scientific name
Broad Bean Mottle Virus (BBMV)
Significance
The virus was seen as being of minor importance until reports of its widespread occurrence began to appear in the 1970s and 1980s.
Symptoms
Plants display leaf symptoms, dwarfing and production of few or no flowers. Infected plants are in concentric patches. BBMV can affect seed quality by causing necrosis and shrivelling of the seed. Seed transmission rates in legumes are low and range from 0.1% to 1.4%.
 Broad Bean Mottle (photo: www.padil.gov.au) |
Hosts
Under experimental conditions susceptibility to infection by virus is found in several families. Susceptible host species are found in the Family Amaranthaceae, Chenopodiaceae, Leguminosae-Papilionoideae, Solanaceae. The following species were susceptible to experimental virus infection: Chenopodium album, Chenopodium amaranticolor, Chenopodium hybridum, Chenopodium quinoa, Cyamopsis tetragonoloba, Glycine max, Gomphrena globosa, Lathyrus odoratus, Lens culinaris, Lupinus albus, Medicago sativa, Melilotus albus, Nicotiana clevelandii, Phaseolus vulgaris, Pisum sativum, Trifolium hybridum, Trifolium incarnatum, Trifolium pratense, Trifolium repens, Trifolium subterraneum, Vicia faba, Vicia villosa.
Geographic distribution
BBMV was reported in faba bean crops in Portugal, Sudan, Morocco, and Algeria. then undertook a regional survey and found BBMV in faba bean crops in Egypt, Morocco, Sudan, Syria and Tunisia. Fortass and Bos (1991) surveyed faba bean crops in Morocco and found that luteoviruses and BBMV were the most prevalent viruses.
Biology and transmission
BBMV survives between growing seasons of the primary pulse host in an alternative host or in infected seed. Due to the fact that the natural host range includes at least four families and includes many food legumes and non-legume wild hosts, it may survive in a range of alternative hosts.
Virus is transmitted by a vector. Virus is transmitted by mechanical inoculation; transmitted by grafting.
Virus is transmitted by arthropods, by insects of the order Coleoptera; Acalymma trivittata, Colaspis flavida, Diabrotica undecimpunctata, Sitona lineata.
Detection/indexing method in place at the CGIAR Center at ICARDA
- Tissue Blot Immuno Assay test (TBIA), Enzyme-linked immunosorbent assay (ELISA)
Treatment/control
- Seed is considered to be the main source of BBMV, therefore sowing virus tested seed is the most effective way of controlling this virus.
- Commercial seed tests are available.
- Chemical control of insects is not an effective method for controlling virus.
Procedure in case of positive test
- Destroying seed, producing healthy seed under controlled conditions
References and further reading
http://www.padil.gov.au/pbt/index.php?q=node/46&pbtID=152
http://www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/00.010.0.03.002.htm
Scientific name
Pea Early-Browning Virus(PEBV)
Other scientific name
Broad Bean Yellow Band Virus (BBYBV)
Significance
Not Significant.
Symptoms
The symptoms are systemic necrotic spots, streaks and mosaic, leaves malformed, plants stunted.
Hosts
Families containing susceptible hosts: Alliaceae, Chenopodiaceae, Compositae, or Cruciferae, Gramineae, Leguminosae-Papilionoideae, Solanaceae. Species inoculated with virus that do not show signs of susceptibility: Allium cepa, Avena sativa, Beta vulgaris, Brassica campestris ssp. rapa, Hordeum vulgare, Lactuca sativa, Lycopersicon esculentum, Medicago sativa, Nicotiana glutinosa, Solanum tuberosum, Trifolium incarnatum, Trifolium pratense, Trifolium repens, Triticum aestivum, Vicia faba.
Geographic Distribution
The virus occurs in Belgium, Italy, Morocco, the Netherlands, Sweden, and the United Kingdom.
Biology and transmission
Virus is transmitted by a vector. Virus is transmitted by mechanical inoculation; transmitted by seeds (37% in Pisum sativum cv. Rondo).
Vector Transmission: Virus is transmitted by nematodes; family Trichodoridae; Trichodorus teres, T. pachydermus in The Netherlands; T. anenome, T. primitivus, T. viruliferus
Detection/indexing method
- Not important (TBIA) Tissue Blot ImmunoAssay test, Enzyme-linked immunosorbent assay (ELISA)
Treatment/control
Not given.
Procedure followed at the centers in case of positive test
Not given
References and further reading
http://www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/00.072.0.01.002.htm
Scientific name
Red Clover Vein Mosaic Virus (RCVMV)
Other scientific name
Pea Stunt Virus
Significance
It reduces the yield of red clover by reducing the foliage growth, decreasing persistence and increasing susceptibility to root rots.
Symptoms
RCVMV is reported to cause stunting in faba beans and inoculated plants have been reported to show chlorotic lesions or general chlorosis, tip mottle and abscission.
Hosts
It also infects a number of temperate pulses including Cicer arietinum (chickpea), Lathyrus odoratus (sweet pea), Lens culinaris (lentil), Pisum sativum (field pea), and Vicia faba (faba bean, broad bean, tick bean). RCVMV is seedborne in Trifolium pratense (red clover), Pisum sativum and Vicia faba.
Geographic distribution
It occurs in North America, South Africa, Europe, and India.
Biology and transmission
- Virus is transmitted by a vector. Virus is transmitted by mechanical inoculation; transmitted by seeds (in Trifolium pratense and Vicia faba, not transmitted by pollen.
- Vector Transmission: Virus is transmitted by arthropods, by insects of the order Hemiptera, family Aphididae; Acyrthosiphon pisum, Myzus persicae, Therioaphis (Pterocallidium) maculatus, Cavariella aegopodii, C. theobaldi. Virus is transmitted in a non-persistent manner.
Detection/indexing method in place at the CGIAR Center at ICARDA
- Not important, (TBIA) Tissue Blot Immuno Assay test, Enzyme-linked immunosorbent assay (ELISA)
Treatment/control
- Use resistant varieties.
- Resistance to vectors is another approach to controlling viruses.
References and further reading
http://www.padil.gov.au/pbt/index.php?q=node/46&pbtID=172
http://www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/00.056.0.04.027.htm
http://www.apsnet.org/online/feature/plantdisease/text/fig08.htm
http://www1.agric.gov.ab.ca/$Department/deptdocs.nsf/All/prm7877?OpenDocument
Comments
- No comments found





Leave your comments
Post comment as a guest