Viruses - groundnut
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
|
Contents: |
Scientific name
Peanut mottle virus (PMoV)
Other scientific names
Peanut green mosaic virus, Peanut chlorotic mottle virus
Importance
High
Significance
PMoV causes substantial yield losses in many parts of the world. In some South East Asian countries losses up to 30-48% have been reported, and in the Indian Sub-Continent the virus is a potential threat to groundnut production (Reddy 1991; Prasada Rao et al. 1996).
Symptoms
Leaf symptoms on groundnuts include mild dark-green mosaic or mottle; leaflet margins can be crinkled and interveinal tissue depressed. Occasional leaf necrosis and deformation, chlorotic spots and stunting are also observed. Infected seeds are often malformed and discolored (Abdelsalam et al. 1987).
Hosts
Arachis chacoense (wild groundnut), Phaseolus vulgaris (common bean), Lupinus angustifolius (lupins), Vigna radiata (mung bean), Pisum sativum (pea), Glycine max (soybean) and forage legumes.
Geographic distribution
PMoV is worldwide in distribution including East Africa, South East Asia, India, Philippines, Taiwan, Malaysia, South America and South East USA (Reddy 1991).
Biology and transmission
PMoV has flexuous, filamentous, non-enveloped particles ranging from 723 to 763 nm in length and 12 nm in diameter (Pietersen and Garnett 1992). Infected groundnuts are considered to be the primary source of PMoV (Prasada Rao et al. 1993) and other nearby leguminous crops become infected from this crop. In addition to being mechanically transmissible, PMoV is also transmitted in a non-persistent manner by several species of aphid, including Aphis craccivora, Aphis gossypii, Hyperomyzus lactucae, Myzus persicae, Rhopalosiphum maidis and Rhopalosiphum padi (Pietersen and Garnett 1992). PMoV is seedborne up to 20% in groundnuts (Bashir et al. 2000). Adams and Kuhn (1977) reported that seed transmission is due to the presence of the virus in the embryo.
Detection/indexing methods at ICRISAT
- Pre export field inspection and ELISA (Sudarshan and Reddy 1989) are used.
Treatment/control
- Not known.
Procedures followed in case of positive test at ICRISAT
- Rejection of seed samples in case of positive test.
References and further reading
Abdelsalam AM, Khalil EM, Fahim MM, Ghanem GA. 1987. The effect of peanut mottle virus infection on growth and yield of peanuts. Egyptian Journal of Phytopathology 19:127-132.
Adams DB, Kuhn CW. 1977. Seed transmission of peanut mottle virus in peanuts. Phytopathology 67:1126-1129.
Bashir M, Ahmad Z, Murata N. 2000. Seed-borne viruses: detection, identification and control, Pakistan Agricultural Research Council, National Agricultural Research Center, Park Road, Islamabad, Pakistan, 156pp.
Pietersen G, Garnett HM 1992. Some properties of a peanut mottle virus (PMoV) isolate from soybeans in South Africa. Phytophylactica 24:211-215.
Prasada Rao RDVJ, Ribeiro GP, Pittman R, Reddy DVR, Demski JW. 1993. Reaction of Arachis germplasm to peanut stripe, peanut mottle and tomato spotted wilt viruses. Peanut Science 20:115-118.
Prasada Rao RDVJ, Chakrabarthy SK, Reddy AS, Girish AG, Mehan VK. 1996. Interception of peanut stripe virus in groundnut germplasm imported from China. Indian Journal of Plant Protection 24:143-145.
Reddy DVR. 1991. Crop profile. Groundnut viruses and virus diseases: distribution, identification and control. Review of Plant Pathology 70: 665-678.
Sudarshana MR, Reddy DVR. 1989. Penicillinase-based enzyme-linked immunosorbent assay for the detection of plant viruses. Journal of Virological Methods 26:45-52.
 Peanut mottle (Peanut mottle virus) of groundnut: symptoms of irregular dark green islands and interveinal depression (photo:ICRISAT) |
Scientific name
Peanut stripe virus (PStV).
Importance
Low
Significance
PStV has been reported to cause about 50% incidence in China ( Xu et al. 1994). In Gujarat, India, the disease incidence has been recorded up to 40% (Varma et al. 1994). In South East Asia, high incidences of up to 38% have been reported in Indonesia (Middleton and Saleh 1988) and the Philippines (Adalla and Natural 1988). PStV infection has a highly variable effect on groundnut yield, depending on the test conditions, cultivar and the virus isolate.
Symptoms
Symptoms on groundnut plants vary, depending on virus isolate and groundnut cultivar. For most isolates, the initial symptoms appear as chlorotic flecks or rings on young quadrifoliates. The plants are slightly stunted. Subsequently, the older leaves show symptoms which are more specific to the isolate: mild mottle, blotch, stripe, chlorotic ring mottle, chlorotic line pattern, oak leaf pattern or necrosis (Wongkaew and Dollet 1990). The symptoms normally persist throughout plant development. The 'stripe' [V-shaped or herringbone pattern] and 'necrotic' isolates, which are seen less often, can severely stunt the plants if they infect them early.
Hosts
Arachis hypogaea (groundnut). Glycine max (soyabean), Lupinus albus (lupine), Medicago sativa (lucerne), Vigna radiata (mung bean), Sesamum indicum (sesame), Vigna unguiculata (cowpea). Cassia occidentalis (coffee senna), Cassia tora (foetid cassia), Centrosema pubescens (Centro), Calopogonium caeruleum, Crotalaria pallida (smooth crotalaria), Desmodium (tick clovers), Indigofera (indigo), Pueraria phaseoloides (tropical kudzu), Cassia obtusifolia (sicklepod) and Stylosanthes biflora (Pencil flower).
Geographic distribution
PStV is widespread in groundnut-growing areas throughout east and south Asia. It was first detected in India in 1987 (Demski et al. 1993. It also occurs in all groundnut growing areas of Indonesia, Malaysia, Myanmar, Philippines, Thailand and Vietnam (Demski et al. 1993).
Biology and transmission
PStV particles are filamentous flexuous rods, approx. 752nm long and 12 nm in diameter. Each particle consists of single protein pieces of 33500 daltons. The genome is single stranded (ss) positive-sense RNA molecule of about 9500 nucleotides. The particles are relatively stable and can be stained with 2% phosphotungstate or ammonium molybdate pH 6.5 (Demski et al. 1993). The virus is transmitted by several species of aphids in a non-persistent manner, which is also the only means of disease spread under field conditions. Aphis craccivora is the major vector for the transmission of PStV. Apart from A. craccivora, Myzus persicae and A. gossypii, and Hysteroneura setariae have been shown to be highly efficient PStV vectors for the transmission of the disease. PStV transmission through groundnut seed can be as high as 37% in artificially inoculated plants (Demski et al. 2004). Under natural conditions, however, the transmission frequency is up to 7%. PStV seed transmission frequency can be influenced by the virus isolate, groundnut cultivar and environment. The virus can be detected in both the embryo and the cotyledon, but not in the seed testa.
Detection/indexing methods at ICRISAT
- Pre export field inspection and ELISA (Sudarshan and Reddy 1989) are used.
Treatment/control
- Not known.
Procedures followed in case of positive test at ICRISAT
- Rejection of seed samples in case of positive test.
References and further reading
Adalla CB, Natural MP. 1988. Peanut stripe virus disease in the Philippines. In: ICRISAT, ed. Coordination of Research on Peanut Stripe Virus: Summary Proceedings of the First Meeting to Coordinate Research on Peanut Stripe Virus Disease of Groundnut, 9-12 June 1987, Malang, Indonesia. Patancheru, Andhra Pradesh, India: ICRISAT, pp9.
Demski JW, Reddy DVR, Sowell Jr. G, Bays D. 2004. Peanut stripe virus - a new seed-borne potyvirus from China infecting groundnut (Arachis hypogaea) . Annals of Applied Biology 105: 495-501.
Demski JW, Reddy DVR, Wongkaew S, Xu Z, Kuhn CW, Cassidy BG, Shukla DD, Saleh N, Middleton KJ, Sreenivasulu P, Prasada Rao RDVJ, Senboku T, Dollet M, McDonald D. 1993. Peanut stripe virus. Information Bulletin No. 38. Patancheru, Andhra Pradesh, 502 324, India: International Crops Research Institute for the Semi-Arid Tropics, Griffin, GA 30223, USA: Peanut Collaborative Research Support Program, 20pp.
Middleton KJ, Saleh N. 1988. Peanut stripe virus disease in Indonesia and the ACIAR Project. In: ICRISAT, ed. Coordination of Research on Peanut Stripe Virus: Summary Proceedings of the First Meeting to Coordinate Research on Peanut Stripe Virus Disease of Groundnut, 9-12 June 1987, Malang, Indonesia. Patancheru, Andhra Pradesh, India: ICRISAT, 4-6.
Sudarshana MR, Reddy DVR. 1989. Penicillinase-based enzyme-linked immunosorbent assay for the detection of plant viruses. Journal of Virological Methods 26: 45-52.
Varma A, Jain RK, Ghewande MP, Nand Gopal V. 1994. Virus dieases of groundnut in India with particular reference to peanut stripe virus. In: Reddy DVR, McDonald D, Moss JP, eds. Working Together on Groundnut Virus Diseases: Summary and Recommendations of International Working Groups on Groundnut Virus Diseases, 15-19 August 1993, Scottish Crop Research Institute, Dundee, UK. Patancheru, Andhra Pradesh, India: ICRISAT, 61-62.
Wongkaew S, Dollet M. 1990. Comparison of peanut stripe virus isolates using symptomatology on particular hosts and serology. Oleagineux (Paris) 45: 267-278.
Xu Z, Zhang Z, Chen K, Reddy DVR, Middleton KJ, Chen J, Wightman JA. 1994. Current research on groundnut virus diseases in China. In: Reddy DVR, McDonald D, Moss JP, eds. Working Together on Groundnut Virus Diseases: Summary and Recommendations of a Meeting of International Working Groups on Groundnut Virus Diseases, 15-19 August 1993, Scottish Crop Research Institute, Dundee, UK; Patancheru, Andhra Pradesh, India: ICRISAT, 59-60.
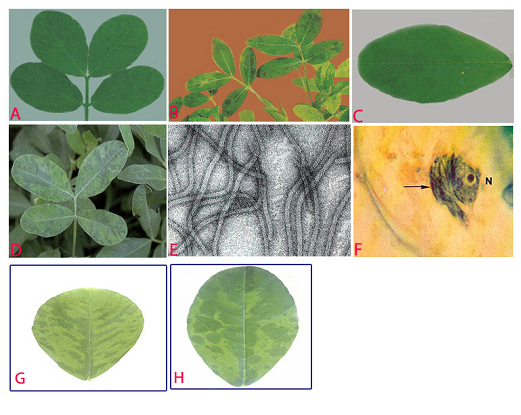
Peanut stripe (Peanut stripe virus) of groundnut: (A)stripe and green banding symptoms; (B)striping and mosaic (oak leaf pattern) symptoms in older leaves; (C)chlorotic flecks; (D)chlorotic ring mottle symptoms; (E)electron micrograph (x 257000); (F)subdivision-IV type inclusive body (arrow) adjacent to the nucleus (N) in PStV infected leaflet; (G)necrotic strips and (H)mild mottle and blotch (photos:ICRISAT) |
Scientific name
Peanut stunt virus(PSV).
Other scientific names
Robinia mosaic virus, Black locust true mosaic virus, Clover blotch virus
Importance
High
Significance
In the 1960s, PSV was a problem in Virginia, North Carolina and Georgia, but it is not of economic importance for groundnut production in the USA. However, PSV sporadically causes a high incidence of peanut stunt disease in Hebei, Henan and Liaoning provinces in China (Xu et al.1992).
Symptoms
Symptomsvary depending on the host plant and the strain of the virus. In groundnuts, there are various degrees of stunting, shortening of the petioles, reduced leaf size, mild mottling and malformation of pods. Seeds from infected plants appear deformed, frequently with a split pericarp wall, and have poor viability.
Hosts
PSV naturally infects several leguminous host species - Arachis hypogaea (groundnut), Vicia faba (broad bean), Glycine max (soybean), Pisum sativum (pea), Vigna unguiculata (cowpea), Lupinus luteus (yellow lupin), Nicotiana tabacum (tobacco), Lycopersicon esculentum (tomato) and Datura stramonium (devils trumpet).
Geographic distribution
PSV is world wide in distribution, including several countries in Europe (France, Hungary, Italy, Poland and Spain; in Asia (China, Georgia, Japan, Korea and India); in Africa (Morocco and Sudan) and also in USA (Subrahmanyam et al. 1992).
Biology and transmission
PSV particles are isometric or polyhedral, with a diameter of ca 25-30 nm. The coat protein of PSV contains a single polypeptide with an apparent molecular weight of about 26 kDa. PSV has a positive-sense tripartite genome (designated RNAs 1, 2 and 3 in order of decreasing size). In addition to the genomic RNAs, the virions also encapsulate a fourth RNA (called RNA 4) which is a subgenomic RNA that functions as mRNA for the viral-coat protein (Naidu et al. 1995 ). Three types of native particle exist, each consisting of the same protein shell, yet containing different RNA species. One type of particle contains genomic RNA 1, another contains RNA 2 and the third contains genomic RNA 3 and subgenomic RNA 4. However, all the particles have the same sedimentation coefficient (S20w). All three genomic RNAs, but not subgenomic RNA 4, are essential for infection. Certain naturally occurring PSV isolates also encapsidate a satellite RNA (satRNA) molecule with its genomic and subgenomic RNAs (Naidu et al. 1995). PSV-associated satRNAs are linear, single-stranded RNA molecules, ranging in size from 391 to 393 nucleotides. PSV satRNA has essentially no sequence homology with its helper virus (i.e. PSV) genomic RNAs (Collmer et al. 1985). Depending on the PSV strain and host species involved, satRNAs can modulate the symptoms caused by PSV (Naidu et al. 1992). PSV supports the replication of its satRNAs but not those associated with cucumber mosaic virus. PSV is transmitted in nature by insect vectors - Aphis craccivora, A. spiraecola and Myzus persicae. It is transmitted in the non-persistent manner. PSV can also be transmitted by mechanical inoculation. PSV is transmitted in a small percentage (0.1%) of groundnut seeds (Kuhn 1969).
Detection/indexing methods at ICRISAT
- Pre export field inspection and double-antibody-sandwich (DAS)-ELISA and an indirect ELISA are used to detect PSV (Bharathan et al. 1984).
Treatment/control
- Not known
Procedures followed case of positive test at ICRISAT
- Incineration of the infected plants and rejection of the infected seeds are applied.
References and further reading
Bharathan N, Reddy DVR, Rajeshwari R, Murthy VK, Rao VR, Lister RM. 1984. Screening peanut germplasm lines by enzyme-linked immunosorbent assay for seed transmission of peanut mottle virus. Plant Disease 68: 757-758.
Collmer CW, Hadidi A, Kaper JM. 1985. Nucleotide sequence of the satellite of peanut stunt virus reveals structural homologies with viroids and certain nuclear and mitochondrial introns. Proceedings of the National Academy of Sciences USA 82: 3110-3114.
Kuhn CW. 1969. Effects of peanut stunt virus alone and in combination with peanut mottle virus on peanut. Phytopathology 59:1513-1516.
Naidu RA, Collins GB, Ghabrial SA. 1992. Peanut stunt virus satellite RNA: analysis of sequences that affect symptom attenuation in tobacco. Virology 189: 668-677.
Naidu RA, Hu CC, Pennington RE, Ghabrial SA. 1995. Differentiation of eastern and western strains of peanut stunt cucumovirus based on satellite RNA support and nucleotide sequence homology. Phytopathology 85: 502-507.
Subrahmanyam P, Wongkaew S, Reddy DVR, Demski JW, McDonald D, Sharma SB, Smith DH. 1992. Field diagnosis of groundnut diseases. Information bulletin no. 36, Patancheru, AP, 502 324, India: International Crops Research Institute for the Semi Arid Tropics. 84pp.
Xu Z, Zhang Z, Chen K, Chen J. 1992. Characteristics of strains of peanut stunt virus by host reactions and pathogenicity to peanut. Oil Crops of China 4: 25-29.
 Peanut stunt (Peanut stunt virus) of groundnut: dwarfing symptoms of branches (photo:ICRISAT) |
Scientific name
Peanut clump virus(PCV).
Other scientific name
Indian Peanut Clump Virus (IPCV)
Importance
High
Significance
PCV infected plants do not produce pods, and yield losses in groundnut grown in light sandy soils are as high as 60 % even in late infected crops (Reddy et al. 1988).
Symptoms
Plants affected by clump disease are conspicuous in the field because of their severe stunting and dark green appearance. Initial symptoms appear on young leaflet as mottling, mosaic and chlorotic rings, but later turn dark green with or without faint mottling as the leaves mature. Early infected plants become severely stunted. Late infected plants may not show concipous stunting but appear dark green with faint mottling on younger leaflets. In late infected plants, clumping may be restricted to few branches. Infected plants become bushy and produce several flowers. Early infected plants may not produce any pods and late infected plants may produce poorly developed pods (Reddy et al. 1988). These plants often occur in patches and the disease reoccurs in the same area of the groundnut field in successive years.
Hosts
PVC causes disease in Triticum (wheat), Hordeum vulgars (barley), Saccharum officinarum (sugarcane), Capsicum spp (chilli), and Cajanus cajan (pigeonpea). It also infects Sorghum bicolor (sorghum), Zea mays (maize), Oryza sativa (rice), Brassica juncea(mustard), Glycine max (soybean) and Vigna radiate (mung bean), but these hosts do not exhibit symptoms (Thouvenel and Fauquet 1981).
Geographic distribution
The disease affects groundnut in several countries in western Africa including Burkina Faso, Ivory Coast and Senegal, and in several countries of Asia, including India and Pakistan, and also in South Africa (Delfosse et al. 1995).
Biology and transmission
IPCV particle dimensions in leaf dip preparations are 184±8 ´ 24±2 nm in uranyl acetate and 169 ± 5 and 239 ± 13 ´ 20±1 nm in phosphotungstate (Thouvenel and Fauquet 1981). In IPCV-Ludhiana strain had 175 nm particles contains RNA-2 (1.35 ´ 106 mol. wt) and 235 nm particles contain RNA-1(1.84 ´ 106mol. wt) since both size of particles are needed to induce local lesions in bean tissues. IPCV isolates from India have been grouped into 3 distinct serotypes - IPCV-H (Hyderabad), IPCV-D (Durgapura) and IPCV-L (Ludhiana). Complementary DNA hybridization tests have shown that isolates D, H and L of IPCV are related to each other but not to furoviruses (Robinson and Reddy 1985). IPCV is seed transmitted up to 11% in groundnut and also through seeds of finger millet, pearl millet, fox tail millet, wheat and maize. IPCV has been transmitted by Polymyxa graminis (Ratna et al. 1991).
Detection/indexing methods at ICRISAT
- Pre export field inspection, and Double antibody sandwich ELISA (DAS-ELISA) and Nucleic acid hybridization (Reddy et al. 1985) tests are used.
Treatment/control
- Seed treatment is not available but satisfactorily controlled by adopting proper cultural practices over a period of time.
Procedures followed in case of positive test at ICRISAT
- Incineration of the infected crop and rejection of the infected seeds are used in case of positive test.
References and further reading
Delfosse P, Bashir M, Malik SN, Reddy AS. 1995. Survey of groundnut viruses in Pakistan. International Arachis Newsletter 15: 51-52.
Ratna AS, Rao AS, Reddy AS, Nolt BL, Reddy DVR, Vijaylakshmi M, McDonald D. 1991. Studies on transmission of peanut clump virus disease by Polymyxa graminis. Annals of Applied Biology 118: 71-78.
Reddy DVR, Robinson DJ, Roberts IM, Harrison BD. 1985 Genome Properties and Relationships of Indian Peanut Clump Virus. Journal of General Virology 66: 2011-2016
Reddy DVR, Nolt BL, Hobbs HA, Reddy AS, Rajeswari R, Rao AS, Reddy DDR, McDonold D. 1988. Clump virus in India: Isolates, host range, transmission and management. In: Viruses with fungal vectors.(Cooper JI, and Asherr, MJCeds) Asso. Appl. Siol. Wellesbourne , UK, pp. 239-246.
Robinson DJ, Reddy DVR.1985. Nucleic acid hybridization tests for relationships among furovirus isolates. In: 5th Ann. Rep., Scottish Crops Research Institute, Invergowrie, Dundee, Scotland, 155.
Thouvenel JC, Fauquet C. 1981. Further properties of peanut clump virus and studies on its natural transmission. Annals of Applied Biology 97: 99-107.
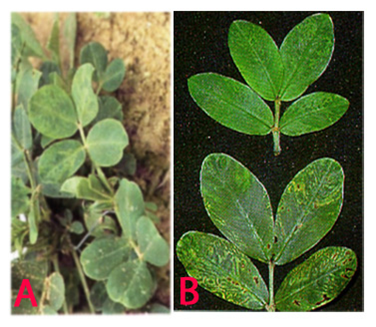 Peanut clump (Peanut clump virus) of groundnut: (A)mosaic and mottling symptoms; (B)darker green and faint mottling older leaves and chlorotic rings (photos:ICRISAT) |
Comments
- No comments found





Leave your comments
Post comment as a guest