Fungi - pearl millet
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
|
Contents: |
Downy mildew; Green ear disease
Scientific name
Sclerospora graminicola (Sacc.) Schroet.
Other scientific names
Scleropthora macrospora
Importance
Medium
Significance
Yield losses upto 60% were reported from India, and several countries in Africa (Singh et al. 1993).
Symptoms
Leaf symptoms begin as chlorosis at the base of the leaf lamina and successive new leaves show a progression of greater leaf coverage. Under conditions of high humidity and moderate temperature, the infected chlorotic leaf area supports a massive asexual sporulation, generally on the abaxial surface of the leaves. Severely infected plants are generally stunted and do not produce panicles. The name 'green ear' stems from the appearance of green panicles due to transformation of floral parts into leafy structures, which can be total or partial (Singh et al. 1993).
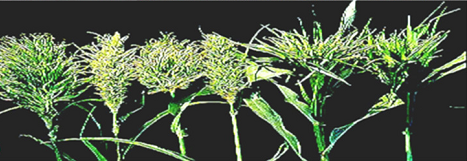 Different malformed green ears (photo: ICRISAT). |
Hosts
S. graminicola is specific to pearl millet.
Geographic distribution
Several countries in Asia and Africa such as Chad, Egypt, Gambia, Malawi, Mozambique, Niger, Nigeria, Zimbabwe, Senegal, South Africa. Also Mali, Burkina Faso, Ivory Coast, Sudan, Kenya, Uganda, Tanzania, Ghana, Togo, Zambia, and a few states in USA.
Biology and transmission
Sclerospora graminicola produces two types of spores, asexual spores known as sporangia, and sexual spores known as oospores. The whitish downy growth of the pathogen on the leaf surface is the “asexual phase”, followed by the “sexual phase” in which oospores are produced within the leaf tissue. Sporangiophores are short, stout, determinate and dichotomously branched structures that emerge from systemically infected leaves through stomata. Sporangia are produced on sterigmata located at the tips of the sporangiophore branches. Sprorangia are hyaline, thin walled, ellipsoid or broadly elliptic and papillate. Sporangia germinate indirectly by producing zoospores. The number of zoospores per sporangium vary from 1-12. Zoospores emerge through a pore produced by the release of the operculum. Zoospores germinate and produce infection in the host. Sexual phase starts with the formation of the oospores in the host. Mature oospores are thick-walled, spherical, and brownish yellow, 22 to 35 um in diameter. Oospores are resting spores and can survive for 8-10 years in field, and cause the primary infection. The pathogen is soilborne and externally seedborne for causing the disease in the succeeding crop season. Secondary spread of the disease occurs through wind borne sporangia (Singh et al. 1993).
Detection/indexing methods At ICRISAT
- Pre export field inspection, and seed washing for oospores detection.
Treatment/control
- Dry seed treatment with metalaxyl @ 2 g a.i. kg-1 seed.
Procedures followed in case of positive test at ICRISAT
- Incineration of infected samples in the field, and rejection of seed samples if oospores are observed under seed washing test.
References and further reading
Ahmed KM, Ravinder Reddy Ch.1993. A Pictorial guide to the identification of seed borne fungi of sorghum, pearl millet, finger millet, chickpea, pigeon pea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the semi-Arid Tropics. 200 pp.
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Singh SD, King SB, Werder J. 1993. Downy mildew disease of pearl millet. Information Bulletin No. 37. Patancheru, AP 502 324, India: International Crops Research Institute for the Semi Arid Tropics. 36pp.
 Downy mildew (Sclerospora graminicola) of pearl millet: (A)chlorosis on leaves; (B)sporangial growth on lower surface of leaf and (C)oospores (photos: ICRISAT). |
Scientific name
Claviceps fusiformis Loveless.
Other scientific names
Claviceps microcephala
Importance
High
Significance
The disease assumes special importance because grain is contaminated by grain-replacing sclerotia which contain alkaloids that affect the health of human beings and animals. Losses in grain yield due to this disease have been estimated as high as 58-70% in F1 hybrids (Thakur and King 1988).
Symptoms
Ergot can be identified when creamy to pinkish mucilaginous droplets called ‘honeydew’ ooze from the infected florets on the panicles. These droplets contain numerous asexual conidia. Within 10-15 days these droplets dry out into hard, dark brown to black structures called sclerotia. These are larger than seed and with a pointed apex, which protrude from the florets in place of the grain.
Hosts
Cenchrus ciliaris (buffel grass)and Panicum antidotale (blue panic grass).
Geographic distribution
The disease is distributed in India, Pakisthan, and several countries in Africa including Botswana, Burkina Faso, Gambia, Ghana, Malawi, Niger, Nigeria, Senegal, Somalia, Tanzania, Uganda, Zambia, and Zimbabwe (Thakur and King 1988).
Biology and transmission
Sclerotia are elongated to round in shape, light pink to dark brown/dark in color, hard to brittle with cavities. These germinate by producing 1-17 fleshy, purplish stipes, 6-26 mm long. Each stipe bears at its apex a globular capitulum which is light to dark brown with numerous perithecial projections. Perithecia are pyriform and are embedded in the somatic tissue in the peripheral region of the capitula. Asci are interspersed with paraphyses in the perithecia and emerge through ostioles. These asci are long and hyaline with apical pores. The thread-like ascospores are hyaline and nonseptate. These cause the initial infection in the field. The fungus produces two types of conidia - macro and micro conidia. Macroconidia are hyaline, fusiform, unicellular, and germinate by producing 1-3 germ tubes from their ends or sides. Microconidia are hyaline, globular, unicellular,, and germinate by producing only one germ tube. Both macro- and microconidia are produced on the tips of the germ tubes that are produced in chains. Disease is spread through soil borne sclerotia, sometimes as contaminated seed with sclerotia from season to season. Secondary spread is occurred through wind born conidia and also through rain splashes and insects (Thakur and King 1988).
Detection/indexing methods at ICRISAT
- Pre export field inspection, and seed examination for detection the sclerotia using magnifying lens.
Treatment/control
- Nil
Procedures followed in case of positive test at ICRISAT
- Incineration of infected samples in the field, and rejection of seed samples if found positive in seed examination.
References and further reading
Ahmed KM, Ravinder Reddy Ch.1993. A Pictorial guide to the identification of seed borne fungi of sorghum, pearl millet, finger millet, chickpea, pigeon pea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the Semi-Arid Tropics. 200 pp.
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Thakur RP, King SB. 1988. Ergot disease of pearl millet. Information Bulletin no. 24. Patancheru, AP. 502324. India: International Crops Research Institute for the Semi Arid Tropics. 24pp.
 Ergot (Claviceps fusiformis) of pearl millet: (A)honydew stage; (B)sclerotial stage and (C)slerotia (photos: ICRISAT). |
Blast; Leaf blast; Brown leaf spot; Pyricularia leaf spot
 Pyricularia Leaf Spot (photo: ICRISAT) |
Scientific name
Pyricularia grisea (Cke.) Sacc.
Other scientific names
Pyricularia penniseti, Pyricularia setariae.
Importance
Medium
Significance
Leaf blast causes early death of seedlings under humid conditions. It causes the discoloration of the forage and can reduce grain and forage production moderate to heavy .
Symptoms
Lesions on foliage are elliptical or diamond-shaped; approximately 2.5-3.5 ´ 1.5-2.5 mm. Lesion centers are grey and water-soaked when fresh but turn brown upon drying. Lesions are often surrounded by a chlorotic halo which will turn necrotic, giving the appearance of concentric rings.
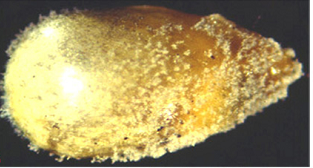 mycelial growth on seed(photo: ICRISAT) |
Hosts
Pennisetum glaucum (pearl millet), Pennisetum purpureum (napier grass).
Geographic distribution
In several countries in the world where warm and humid conditions prevail over a period blast appears on pearl millet.
Biology and transmission
Conidia are pyriform, hyaline, and mostly 3-celled with a small appendage on the base cell. Conidia measure approximately 17.5-30.8 ´ 5.9-8.8 mm (Mehta et al. 1953). Germination, appresoria formation, and invasion of host cells are more at 25°C (Yadava and Agnihotri 1980). Transmission occurs through windborne conidia. It is also reported as seed borne (Singh and Pavgi 1977).
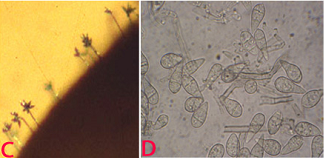 (C)conidiophore with conidia on seed and (D)conidia. (photos: ICRISAT) |
Detection/indexing methods at ICRISAT
- Pre export field inspection and blotter test.
Treatment/control
- Not known
Procedures followed in case of positive test at ICRISAT
- Incineration of infected samples in the field, and rejection of seed samples if found positive in blotter test.
References and further reading
Ahmed KM, Ravinder Reddy Ch.1993. A Pictorial guide to the identification of seed borne fungi of sorghum, pearl millet, finger millet, chickpea, pigeon pea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the semi-Arid Tropics. 200 pp.
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Mehta PR, Singh B, Mathur SC. 1953. A new leaf spot disease of bajra (Pennisetum typhoides Staph and Hubbard) caused by a species of Pyricularia. Indian Phytopathology 5:140-143.
Singh DS, Pavgi MS. 1977. Perpetuation of Pyricularia penniseti causing brown leaf spot of bajra. Indian Phytopathology 30: 242-244.
Yadava RKS, Agnihotri JP. 1980. Epidemiology of Pyricularia leaf spot of bajra. Indian Phytopathology 33:150.
Scientific name
Moesziomyces penicillariae (Bref.) Vanky.
Other scientific names
Tolyposporium penicillariae, Tolyposporium senegalense
Importance
High
Significance
In general, grain loss of 5-20% has been reported (Chahal et al. 1994).
Symptom
In the infected florets, the ovaries are converted into sori. The sori are larger than grains and appear as enlarged, oval to conical bodies projecting beyond the glumes in place of grains. Initially the sori are bright green but later turn brown to black. When the sori mature the membrane rupture and release brown to black mass of spores (Thakur and King 1988).
 Smut (Moesziomyces penicillariae) of pearl millet: (A)smut sori on panicles (photo: ICRISAT) and (B)sori with teliospores (photo: Chahal et al.1994)/ |
Host
Specific to pearl millet.
Geographic distribution
USA, India, Zimbabwe, Senegal, Chad, Niger, Nigeria, Zambia, Sudan, Cameroon, Burkina Faso, Ghana, Mali, Tanzania (Rachie and Majmudar 1980).
Biology and transmission
The teleutospores occur in compact, ball-like masses called spore balls. Spore balls are circular to near polyhedral. The number of teleutospores aggregated in balls varies from 200 to 1400. Teleutospores are mostly angular to round, light brown in color and 7-12 mm in diameter. Teleutospores germinate sporadically and produce 4 celled promycelium laterally and terminal. Variation in germination patterns of teleutospores occurs while they are held in the spore balls, and the sporidia are produced on branched hyphae in chains (Thakur and King 1988). These sporidia are the main propagules for the cause of the disease. Mostly the teleutospores are wind borne for the secondary spread of the disease. Sometimes the teleutospores are attached to the surface of the seed and act as primary source of inoculum. Teleutospores are also soilborne.
 (C)spore masses and (D)spore balls (photos: ICRISAT). |
Detection/indexing methods at ICRISAT
- Pre export field inspection and seed washing test. A new method is developed for elimination of spores from contaminated seed.
Treatment/control
- Spore balls (teleutospores) have been removed by salvaging the smut-contaminated seeds by mixing with sand and ethanol for 3 min while stirring.
Procedures followed in case of positive test at ICRISAT
- Incineration of infected samples in the field, and rejection of seed samples with positive test under seed washing test.
References and further reading
Ahmed KM, Ravinder Reddy Ch.1993. A Pictorial guide to the identification of seed borne fungi of sorghum, pearl millet, finger millet, chickpea, pigeon pea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the semi-Arid Tropics. 200 pp.
Chahal SS, Thakur RP, Mathur SB. 1994. Seed borne diseases and seed health testing of pearl millet. Danish Government Institute of Seed Pathology for developing countries, Copenhagen, Denmark. 72pp.
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Rachie KO, Majmudar JV. 1980. Pearl millet. University Park, Philadelphia, USA: Pennsylvania University press. 307pp.
Thakur RP, King SB. 1988. Smut disease of pearl millet. Information Bulletin No. 25. Patancheru, AP 502 324, India: International Crops Research Institute for the Semi-Arid.
Scientific name
Bipolaris setariae (Saw.) Shoem. [anamorph]
Cochliobolus setariae [Teleomorph]
Other scientific names
Drechslerasetariae
Importance
Medium
Significance
Infection at seedling stage results in death of plants and reduces crop stand in the field (Shetty et al. 1982). Infected plants produce discolored grains and seed of poor quality (Kameswara Rao et al. 2002).
Symptom
Foliar symptoms vary, as brown flecks, fine linear streaks, small oval spots; large irregular oval, oblong, or almost rectangular spots measuring 1-10 ´ 0.5-3 mm. Large fusiform lesions are sometimes produced. Lesions may expand and coalesce. Lesions may be solid dark brown but usually become tan or grayish brown with distinct dark brown border (Luttrell 1954).
 Leaf spot (Bipolaris setariae) of pearl millet: (A)leaf spots (photo: Chahal et al.1994); (B)infected seed and (C)fungal growth on seed (photos: ICRISAT). |
Host
Pennisetum glaucum (pearl millet), Pennisetum purpureum (napier grass), Panicum fasciculatum Swartz (browntop millet), Saccharum officinarum (sugarcane), Zea mays (maize), Sorghum bicolor (sorghum), Paspalum scrobiculatum (little millet), Panicum miliaceum (kodo millet), Hordeum vulgare (barley), Triticum aestivum (wheat), Avena sativa (oat), Imperata cylindrica (Cogongrass) and Cynodon dactylon (bermudagrass).
Geographic distribution
Drechslerasetariae is worldwide in distribution including USA, Hawaii, India, Japan, Zimbabwe and Zambia.
Biology and Transmission
The mycelium is dark and individual hyhae are irregularly branched with rough surfaces.Conidiophores are mostly hypophyllous, simple, 2-8 septate, erect, cylindrical, brown, slightly swollen at the base and geniculate at the apex. They are 72-199 mm in length and 5.6 to 9 mm in width. Conidia are acrogenous, 39-12 mm, 4-10 septate, ellipsoid, straight or slightly curved, pale to moderately dark brown and thin walled (Chahal et al. 1994). The pathogen is both soil borne and seed borne.
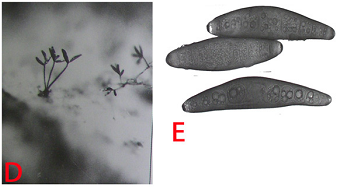 (D)conidiophores with conidia and (E)conidia (photos: ICRISAT). |
Detection/indexing methods used in CGIAR at ICRISAT
- Pre export field infection and blotter test (Girish et al. 2001).
Treatment/control
- Rejection of the seed samples.
Procedures followed in case of positive test at ICRISAT
- Incineration of infected samples in the field, and rejection of seed samples if found positive under blotter test.
References and further reading
Ahmed KM, Ravinder Reddy Ch.1993. A Pictorial guide to the identification of seed borne fungi of sorghum, pearl millet, finger millet, chickpea, pigeon pea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the semi-Arid Tropics. 200 pp.
Chahal SS, Thakur RP, Mathur SB. 1994. Seed borne diseases and seed health testing of pearl millet. Danish Government Institute of Seed Pathology for developing countries, Copenhagen, Denmark. 72pp.
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Girish AG, Singh SD, Chakrabarty SK, Prasada Rao R.D.V.J, Surender A, Varaprasad KS, Bramel PJ. 2001. Seed microflora of five ICRISAT mandate crops. Seed Science and Technology Journal 29: 429-443.
Kameswara Rao N, Bramel Cox P, Reddy KN, Singh SD, Girish AG, Appa Rao S, Mahalakshmi V. 2002. Optimizing seed quality during germplasam regenration in pearl millet. Genetic Resources and Crop Evolution 49: 153-157, Kluwer Academic Publishers, Printed in Netherlands.
Luttrell ES. 1954. Diseases of pearl millet in Georgia. Plant Disease Reporter 38: 507-514.
Shetty HS, Mathir SB, Neergaard P, Safeeula KM. 1982. Drechslera setariae in Indian pearl millet seeds, its seed-borne nature, transmission and significance. Transactions of the British Mycological Society 78: 170-173.
Comments
- No comments found





Leave your comments
Post comment as a guest