CGKB News and events Procedures
Please click on the boxes in the diagram below or use the menu on the left to go to the topic of your interest.

Chapter 17: Plant health and germplasm collectors
R. Macfarlane
Whitby Porirua, New Zealand
E-mail:bob.macfarlane(at)maf.govt.nz
G. V. H. Jackson
Queens Park Sydney, Australia
E-mail: gjackson(at)zip.com.au
E. A. Frison
Bioversity International, Rome, Italy
E-mail: e.frison(at)cgiar.org
|
2011 version |
1995 version |
||
|
Open the full chapter in PDF format by clicking on the icon above. |
|||
This chapter is a synthesis of new knowledge, procedures, best practices and references for collecting plant diversity since the publication of the 1995 volume Collecting Plant Genetic Diversity: Technical Guidelines, edited by Luigi Guarino, V. Ramanatha Rao and Robert Reid, and published by CAB International on behalf of the International Plant Genetic Resources Institute (IPGRI) (now Bioversity International), the Food and Agriculture Organization of the United Nations (FAO), the World Conservation Union (IUCN) and the United Nations Environment Programme (UNEP). The original text for Chapter 17: Plant Health and Germplasm Collectors, authored by E. A. Frison and G. V. H. Jackson has been made available online courtesy of CABI. The 2011 update of the Technical Guidelines, edited by L. Guarino, V. Ramanatha Rao and E. Goldberg, has been made available courtesy of Bioversity International.
Please send any comments on this chapter using the Comments feature at the bottom of this page. If you wish to contribute new content or references on the subject please do so here.
Back to list of chapters on collecting
Internet resources for this chapter
Abstract
When plant germplasm is moved nationally or internationally, there is a risk of the concomitant movement of pests (insects, pathogens, weeds and other organisms). Further, the quality of samples may be compromised by pests affecting viability during storage or, later, during multiplication and characterization.
This chapter suggests what collectors need to do to minimize these dangers. It is divided into (1) what to do when planning collecting missions (assembling the pest information, plant-health documents, intermediate quarantine, pest identification), (2) what to do in the field (minimizing the pest risk, record keeping, preservation of pest specimens), and finally, (3) what to do back at the base (preparing samples for inspection, phytosanitary treatments and certification, documents, and preparation of pests for identification). If followed systematically, these guidelines will facilitate plant germplasm reaching its destination unhindered, and will contribute to its value.
Introduction: the need for healthy germplasm
Uncontrolled movement of plant germplasm between countries spreads pests, but regulation of the movement of plant germplasm can help to reduce these risks. Pests, as defined by the International Plant Protection Convention (lPPC) (https://www.ippc.int/index.php?id=1110589&L=0), are “any species, strain or biotype of plant, animal or pathogenic agent injurious to plants or plant products” (IPPC 2010). (The IPPC facilitates cooperation between contracting parties to protect the world's cultivated and natural plant resources from the spread and introduction of pests of plants, while minimizing interference with the international movement of goods and people.)There are those that can be easily seen with the naked eye, such as insects, mites, slugs and snails, rats, plants and seeds, and those that are microscopic, such as fungi and the like, bacteria, phytoplasma, viruses and viroids. Previously, pests were considered to be only those organisms potentially damaging to crops, but now the term pest also includes newly introduced organisms that might damage ecosystems and plant and animal biodiversity.
Because there are dangers inherent in the movement of plant germplasm, most countries have legislation to regulate the entry (and sometimes the internal movement) of plants, plant parts and their products. In particular, the movement of wild collected germplasm causes significant concern, as its pest status is likely to be poorly known. Consignments of germplasm arriving in the importing country without proper documentation will be treated, reshipped or destroyed, irrespective of their botanical significance, the type of pest infestation or the status of the collector. To reduce the risk of accidental transfer of pests, germplasm should always be collected, processed and shipped in compliance with the phytosanitary requirements of the importing country. Contact details of most countries’ plant quarantine services can be found on the IPPC website.
It is possible, from long experience and sheer weight of knowledge of a plant species, that collectors might be better informed about the possible presence of quarantine pests associated with plant germplasm than the authorities in the importing country. In such cases, they should divulge this information, while at the same time ensuring that the germplasm collected and dispatched is free of pests to the extent that is possible.
There are other, perhaps less obvious, reasons why pests should be given attention when germplasm is collected. Pests might affect the quality, and therefore the usefulness, of germplasm samples. Infection by pathogens can reduce the viability of seeds during storage. When material is multiplied, growth may be distorted, colours altered and disease susceptibility increased. These changes may make it difficult, if not impossible, to collect characterization and preliminary evaluation data, and some important characteristics, crucial for plant-improvement schemes, might go undetected. In addition, infested samples are unlikely to be distributed. They cannot be grown out and regenerated and, if stored, they will remain unused and will deteriorate.
It is, therefore, important to know what pests are likely to be associated with the target gene pool. This will allow an assessment of the risks associated with moving the germplasm, as well as providing for appropriate measures to be devised to reduce the risk to a minimum. It is also important to document any pests present on the target species at the time of collecting. This information, part of the passport data of the sample, will improve the usefulness of the germplasm and will also help during quarantine examination.
For all these reasons, it is often useful to include a plant-protection specialist in collecting teams if funds and logistical considerations allow. Preferably, this should be a plant pathologist experienced in the species to be collected, as pathogens are more difficult than insects and mites to detect during collecting and to eradicate from plant samples. If a plant-protection specialist cannot participate in the collecting mission, collectors should become familiar with the major pests of the target species. In all cases, collectors will have to ensure that the phytosanitary requirements of the importing country have been met and proper documentation has been assembled so that plant samples reach their intended destination unhindered.
This chapter gives guidelines on how these issues may be addressed. It considers what must be done at the planning stage, while collecting in the field and, finally, just before samples are dispatched.
Planning the collecting mission
At the planning stage, attention must be given to the pests that might be encountered on the target species, and to the importing country’s regulations governing plant movement. The following questions need to be considered when assembling information on plant pests:
-
What pests have been recorded on the target species in the country of collecting, especially in the target area?
-
What plant parts are they found on?
-
How are the pests transmitted?
The following questions need to be answered to ensure compliance with phytosanitary regulations:
-
What is the final destination(s) of all samples, including subsamples?
-
What are the phytosanitary import requirements of the country(ies) of destination?
-
What are the procedures for obtaining a phytosanitary certificate in the country of collecting?
-
What are the procedures for verifying that the phytosanitary requirements of the importing country have been met prior to export?
Assembling information on pests
Since the first edition of this document was published, there has been an enormous change in the availability of information on plants and other organisms. The internet now allows immediate access to the most up-to-date information available in publications, research centres and museums worldwide. Perhaps the first point of contact for a collector seeking information on a plant or pest is to run a search for a pest under its presently accepted name (and at least one of its synonyms) through a search engine such as Google, Bing or Yahoo. This will generally turn up several significant leads for further enquiry, often with researchers familiar with the species being searched.
There are now literally hundreds of databases publicly available over the internet, and many others with restricted access for either commercial or confidentiality reasons. A selection of these is provided in the reference section, below, along with a selection of texts that may be consulted for information on the pests of specific crops. For example, the American Phytopathological Society publishes a particularly useful set of documents on the identification of crop plant diseases (www.cplbookshop.com/glossary/G583.htm).
On pest distributions, the following are important sources for accurate data on the worldwide distribution of plant pests and diseases of economic or quarantine importance:
|
Distribution maps of pests |
lIE (1968 et seqq.) |
|
|
Distribution maps of plant diseases |
IMI (1942 et seqq.) |
Collectors should confirm with the relevant institutes that these maps contain the most up-to-date information. Detailed descriptions, including notes on the transmission of many of the pests figured in the maps, can be sought from the following publications:
|
Descriptions of fungi and bacteria |
IMI (1964 et seqq.) |
|
|
Descriptions of plant-parasitic nematodes |
lIP (1972 et seqq.) |
|
|
Descriptions of plant viruses |
CMIIAAB (1970-1984) AAB (1985 et seqq.) |
The CABI Crop Protection Compendium (CPC) (www.cabi.org/cpc) is also a useful source of information on some 3000 pests, diseases, natural enemies and crops (with 400 recently commissioned sheets added in 2010) and basic information on 27,000 more species. The CPC helps with pest identification and distribution (including maps) and with phytosanitary and quarantine issues.
On viruses, CABI and the Australian National University have collaborated on a major database: the Virus Identification Data Exchange (VIDE). Viruses of Tropical Plants (Brunt et al. 1990) is an output of the database, as is Plant Viruses Online: Descriptions and Lists from the VIDE Database (Brunt et al. 1996). This site also provides links to some excellent web sites on plant viruses.
The Consultative Group on International Agriculture Research (CGIAR) supports a consortium of research centres around the world, many of which focus on specific crop plants:
-
Africa Rice Center
-
Bioversity International
-
International Center for Tropical Agriculture (CIAT)
-
Center for International Forestry Research (CIFOR)
-
International Maize and Wheat Improvement Center (CIMMYT)
-
International Potato Center (CIP)
-
International Center for Agricultural Research in the Dry Areas (ICARDA)
-
International Crops Research Institute for the Semi-Arid Tropics (ICRISAT)
-
International Institute of Tropical Agriculture (IITA)
-
International Rice Research Institute (IRRI)
-
World Agroforestry Centre (ICRAF)
Many of these research centres publish useful illustrated guides to the pests of their mandate crops. These are particularly useful in the field.
The CGIAR also has an online portal, the Crop Genebank Knowledge Base (http://cropgenebank.sgrp.cgiar.org/index.php?option=com_content&view=article&id=137&Itemid=238&lang=english), with guidelines for the safe transfer of germplasm for 15 seed crops and five clonally propagated crops under the mandate of CGIAR germplasm banks. In the introduction, the website states that it “summarizes information on current practices and guidelines for the safe transfer of germplasm gathered from the seed and crop health laboratories from CGIAR Centres in charge of the different crops”.
For each of the crops of interest there are sections on (1) germplasm import and export requirements, (2) technical guidelines for the detection and treatment of pests and pathogens and the safe transfer of germplasm and (3) best practices in place at the CGIAR Centres.
In addition, the Food and Agriculture Organization of the United Nations (FAO) and Bioversity International have published a series of booklets of crop-specific technical guidelines for the safe movement of germplasm (published under Bioversity’s previous names: International Board of Plant Genetic Resources and International Plant Genetic Resources Institute). They describe technical procedures that minimize the risk of introducing pests with the movement of germplasm for research, crop improvement, plant breeding, exploration or conservation. The recommendations in these guidelines are intended for germplasm for research, conservation and basic plant breeding programmes; they are not meant for trade and commercial consignments concerning the export and import of germplasm. Each booklet is divided into two parts: the first makes recommendations on how best to move the germplasm of the crop concerned and lists institutions recovering and/or maintaining healthy germplasm, the second covers the pests and diseases of quarantine concern, giving a description of therapy and indexing methodologies. So far, guidelines have been produced for the following crops (full citations and URLs are provided in the reference section below):
|
Acacia spp. |
Musa spp. |
There are many older texts that have crop-by-crop analysis of the problems and risks attendant on the transfer of plant germplasm, such as Hewitt and Chiarappa (1977), and these, too, can often contain information that remains useful.
Finally, pest surveys might also be consulted, if available, to determine which pests have been recorded on the target species in the collecting region. However, in many countries, such surveys are far from complete: sometimes, they have not been done at all, are outdated or do not cover the entire country, concentrating on the more easily accessible areas. Another problem is that pest surveys tend to record pests of crop plants, neglecting wild relatives, and rarely include native plants. This lack of information is a major barrier to the formulation of quarantine regulations appropriate to the exchange of germplasm of many plant species, including crop species.
Assembling the required plant health documents
It is essential to begin making phytosanitary arrangements early in the planning phase of any collecting expedition. Delays in obtaining the appropriate documents are common, but without these documents, the mission might have to be postponed or, worse, the samples destroyed. It is the responsibility of collectors to obtain the necessary documents in order to transfer plant germplasm. Two documents are commonly required for international plant transfer: an import permit and a phytosanitary certificate.
The import permit
The import permit must be obtained from the country or countries of destination of the germplasm well before the mission sets out. Information is also needed on how to obtain a phytosanitary certificate in the country of collecting and whether other authorizations are required to export germplasm, such as authorization under the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), which is an international agreement between governments that aims to ensure that international trade in specimens of wild animals and plants does not threaten the survival of these species. The FAO/IPPC Secretariat has a list of plant-protection services worldwide with contact addresses of the authorities responsible for issuing these documents. This list is available online at https://www.ippc.int/index.php?id=1110520&no_cache=1&type=contactpoints&L=0.
Regulations differ among countries according to the perceived risks involved in making the importation; the conditions of entry will be detailed on the import permit. Treatments may be required both in the country of export and in the country of import. If sub-samples are to be sent to several countries, permits must be obtained from each one. When no conditions apply and germplasm is allowed unconditional entry, it is advisable to obtain a document from the plant-protection service of the importing country to that effect. This will facilitate inspections at the border.
Usually, two copies of the import permit are provided. The top copy should accompany the consignment (if there is to be only one) and the other copy should be retained by the collector. A photocopy is usually allowed for multiple consignments.
It is recommended that collectors advise the importing phytosanitary border authorities of the number and approximate size of samples well ahead of arrival, so that the quarantine inspection service can plan to process the samples quickly. If the plants are to be grown in post-entry quarantine in the importing country or in a third country, then arrangements for this must be made during the planning phase of any expedition to ensure that facilities and space are available when needed.
The phytosanitary certificate
The phytosanitary certificate is issued by the quarantine authority of the exporting country, certifying that the product meets the phytosanitary regulations of the importing country. Consignments are inspected and the certificate issued if they are “free from quarantine pests and practically free of injurious pests” (see the IPPC model phytosanitary certificate, appendix 17.1 at the end of this chapter). A “quarantine pest” is different from a merely “injurious pest” in this statement in that it is of potential national economic importance to the country and not yet present there, or present but not widely distributed, and being actively controlled (IPPC 2010).
In some instances, in order to reduce the overall pest risk, germplasm consignments will need to be given phytosanitary treatments in the country of origin (see chapter 20 on the potential risks for seed viability of such treatments). Fumigation may be requested or the samples may be dipped or dusted in an insecticide or fungicide, given a hot-water treatment, or whatever is considered appropriate by the importing country. The treatments should be applied exactly as requested. If the collector is not confident that a treatment will be applied correctly in the exporting country and that it might fail to control the pest or that the germplasm might be damaged, it may be possible to negotiate with the importing country to have the treatment done under secure quarantine after arrival there. The permit may seek “additional declarations” verifying that these treatments have been applied as required. These, as well as details of the treatments, must be specified/declared on the phytosanitary certificate. Finally, the certificate should be signed by the authorized government representative.
Under no circumstances should alternative treatments to those specified on the import permit be applied without first requesting the authority of the importing country. Alternative treatments may be ignored by the importing quarantine inspector and a second treatment applied, which could reduce the viability of the germplasm. Likewise, if no treatments are requested, none should be given, since importing countries may wish to inspect or test germplasm consignments, and treatments already applied to seeds, for instance, may mask symptoms of seed-borne pathogens and interfere with laboratory tests. If seeds are treated prior to entry, contrary to the conditions of the permit, this could seriously jeopardize their importation.
Where germplasm samples are to be sent to more than one country, it is necessary to obtain phytosanitary certificates that comply with the requirements of each destination. It is important that the certificate(s) should be issued without amendment or erasure. Many countries refuse to accept altered certificates.
A fee may be charged for fumigation or disinfection treatments and, occasionally, for inspection.
Two copies of the phytosanitary certificate should be obtained, if possible. If there is only one, then the collector should make a copy. The original phytosanitary certificate should accompany the consignment and the copy should be kept with the other records of the collecting mission.
Documentation and intermediate quarantine
Collectors are responsible for arranging the documentation for germplasm samples that have to be grown in intermediate (third-country) quarantine. Such arrangements are necessary when it is unsafe to make transfers directly to the importing country. Procedures are essentially similar to those outlined above: an import permit must be obtained from the quarantine authority of the intermediate country. A copy of this should accompany the consignment, together with the phytosanitary certificate showing any treatments or endorsements requested on the permit. After the samples have been grown in intermediate quarantine and declared safe for further transfer, an import permit must be obtained from the country of final destination and a new phytosanitary certificate issued by the intermediate country.
Planning the identification of pests
Misidentifications of pests can seriously jeopardize the usefulness of consignments. Identification services for fungi, bacteria, nematodes are provided by CABI Global Plant Clinic (www.cabi.org/default.aspx?site=170&page=1017&pid=2301).
Costs vary depending on whether or not a country is a member of CABI. CABI also publishes useful directories of organizations, such as the International Mycological Directory (Hall and Hawksworth, 1990). It may be possible to arrange with the Danish Government Institute of Seed Pathology for Developing Countries (in Hellerup, Denmark) for the identification of important seed-borne diseases of tropical countries.
Identification of virus and virus-like infections is more problematical. Specimens need to be sent to institutes specializing in particular crop plants. Lists of institutes providing this service (such as the Tropical Virus Unit at the Institute of Arable Crops Research, Rothamsted Experimental Station, UK) can be found in the appropriate booklet in the FAO/IPGRI series of safe transfer guidelines. CABI also gives advice.
Identification of arthropods and many other biota can be obtained at a cost through the Natural History Museum, South Kensington, UK (www.nhm.ac.uk/about-us/contact-enquiries/identification-and-general-science-enquiries/index.html), and many other museums. In all cases, arrangements must be made well ahead of dispatch to allow the orderly processing of specimens. Import permits may be needed. If so, these must be obtained from the appropriate authorities in the country where specimens are to be examined. Collectors should ensure that the institutes making the identifications know where to send the results.
In the field
Minimizing the pest risk
Familiarity with the symptoms caused by pests and with which plant parts are most likely to be contaminated by the pests of concern is essential. In general, the risk of spreading pests with germplasm is greatest if rooted plants are moved. This is because of the likelihood that nematodes and other soil-borne pathogens will be present; these are difficult to treat without destroying the plant tissues. Other types of vegetative propagating material (e.g., stems, bulbs, corms, etc.) also present a risk, mainly because of infection from systemic pathogens. The international movement of seeds and pollen is considered safer, as fewer pests are harboured by these plant organs. Phytosanitary considerations may therefore contribute to the decision as to what plant part(s) to collect.
It may be possible to apply curative treatments to lessen or eradicate the pest risk. For surface-borne pathogens and insects, pesticide treatments and fumigation may be tried. Where virus, virus-like organisms and internally borne fungi and bacteria are a threat, thermotherapy and shoot-tip culture are most appropriate.
For vegetatively propagated species, transfer of germplasm as in vitro cultures will greatly reduce the pest risk. Nevertheless, it should be stressed that in vitro cultures do not eliminate the risk entirely. They should be complemented by indexing (testing) for viruses and virus-like organisms that are likely to be present in the area where the germplasm was collected.
The technical guidelines for the safe transfer of germplasm give general advice to collectors on the type of germplasm considered safe to move internationally, as well as detailed technical recommendations on how the germplasm may be treated to ensure that it is free of pests. In some instances, because of the severity of the pest and the difficulty of collecting healthy material from the field, the guidelines advise on transfer of material through a third country, where therapy and indexing procedures can be carried out to ensure freedom from internally borne pathogens. The general recommendations of the guidelines are useful even for crops not specifically covered in the series to date.
Recording data on pests
It is important for collectors to record the pests present on their target species and to note whether other pests are present in the target region. Noting that plants are free of pests in an area where pests are common is equally important. Collectors should attempt to describe the symptoms caused by pests. It is, however, often difficult for someone untrained in plant pathology or entomology to do this. Symptoms may be caused by a combination of several pests, or the causal agent may be obscured by the presence of a minor one or by an opportunistic saprophyte. Symptoms due to root attack or internal pathogens are often particularly difficult to interpret. Where there is doubt as to the identification of pests, plant specimens showing typical symptoms should be collected and dried or preserved by other means, as appropriate (see below).
A description of symptoms should include information on the following (Sonoda 1979):
-
the general condition of the plant
-
the plant part(s) affected
-
the type of damage
-
the stage of growth affected
Rating the severity of attack, both in terms of its effect on the individual plant(s) affected and in terms of the percentage of the population affected, will increase the value of the information. Descriptor lists are published by Bioversity International for many crops, cataloguing the important pests and giving scales of severity. These are available online at www.bioversityinternational.org/research/conservation/sharing_information/descriptor_lists.html.
Colour photographs showing the full range of symptoms, including close-ups of damaged areas and of the pests themselves, are often useful diagnostic tools (Sonoda 1979).
Farmers' knowledge of pests can be extensive and detailed. Some examples are given by Altieri (1993). Collectors can often complement the kinds of observations described above with discussions with knowledgeable local people.
Preservation of pests associated with germplasm samples
Correct identification of pests depends on the quality of the specimens prepared in the field. Collectors should be equipped at least with specimen bottles, alcohol (75% isopropyl alcohol) and formalin for preserving insects, mites and nematodes, and with newspapers and plant presses for making dried herbarium specimens of plants with fungal and bacterial diseases (chapter 27). Specimens may need to be shared among several institutes, and sufficient material should be collected to allow this.
Sonoda (1979) gives guidelines on capturing, killing and storing insects and other pests in the context of germplasm collecting. For insect pests, representative specimens of all life stages may be necessary for taxonomic identification. Insects can be captured using nets, by beating plants over a cloth or by using an aspirator. They can be killed using potassium cyanide or ethyl acetate, both of which are dangerous and should be clearly labelled and stored properly. Some insects must be pinned (e.g., beetles, flies and wasps); others can be stored in alcohol (e.g., caterpillars and other larvae, ants, aphids, scales and mealybugs) and others can be stored in small envelopes (e.g., moths and butterflies).
Dried specimens of diseased plants should include as much of the plant as possible, showing both old and new lesions. Fresh specimens can also be collected and stored in plastic bags. They will remain useful longer if refrigerated. Fungal and bacterial pathogens may be isolated from diseased plants in the field, but this requires sterile techniques and is not often feasible in the context of plant germplasm collecting.
Plants infected with viruses or virus-like organisms present the collector with the greatest challenge, as the material needs to be processed in different ways according to the type of pathogen. Where tissues are thought to contain non-cultivable mollicutes (formerly referred to as mycoplasma-like organisms), they need to be fixed in glutaraldehyde; whereas, tissues for virus examination may be sent fresh, dried as thin (1mm x 10mm) sections over calcium chloride (or silica gel) or as sap stained on electron microscope grids. Because of the complexity of the subject, it is essential that, prior to departure, collectors seek advice on the preservation of specimens from the institutes where the specimens are to be sent for examination.
Details of methods of preserving various kinds of diseased material can be found in The Plant Pathologist's Pocketbook (Waller et al. 2001). Methods for collecting and preserving different insect groups can be found in Bland and Jacques (1978), Borror et al. (1976) and British Museum (Natural History) (1974).
Back at base: treatment and dispatch of germplasm samples
This section gives a summary of the phytosanitary procedures involved in handling plant germplasm after it has been collected, along with brief notes on the dispatch of specimens for pest identification. For other aspects of the tasks that will need to be undertaken once back at base, see Chapter 28.
Inspection
Missions should carefully prepare germplasm samples before they are presented to quarantine authorities for inspection, treatment and certification.
-
Germplasm samples should be carefully inspected for pests, insects and mites as well as for lesions or colour patterns that might denote fungal, bacterial or viral pathogens. Where such pests, or symptoms of pests, are present, the pests and/or the symptom-bearing seeds should be removed.
-
Bare-rooted plants should be thoroughly washed to ensure they are free of soil, which might harbour nematodes and other soil-borne pathogens.
-
Seeds and pollen should be free of debris. If debris is present, it should be removed.
Phytosanitary treatments and certification
-
If mandatory treatments are prescribed on the import permit or endorsements are required, these should be given by the relevant government authority exactly as requested.
-
After treatments have been applied, they should be detailed on the phytosanitary certificate, together with any other endorsements requested by the importing country.
-
The phytosanitary certificate should bear the stamp of the organization issuing the certificate and should be signed by an authorized officer. Many countries are now issuing phytosanitary certificates electronically with stamps and signatures added in the computer (known as ePhytos). These are equally valid and may ease on-arrival arrangements because they are increasingly sent to the importing country digitally soon after they are issued.
-
Collectors should ensure that the phytosanitary certificate contains the following information:
-
name and address of the exporter
-
name and address of the consignee
-
number of samples of each species in the consignment
-
botanical name of each species
-
details of any phytosanitary treatments applied
-
additional endorsements required by the import permit
-
Documents accompanying germplasm consignments
-
The original phytosanitary certificate, plus a copy of the import permit, should accompany each consignment. Most importing countries will allow photocopies of import permits if there are multiple shipments, but it would be best to confirm this from the quarantine authorities of the importing country if there are any doubts. A copy of the import permit should be placed on the outside of the package so it can be forwarded to the plant quarantine authorities without the need to open it. A photocopy of the permit should be included inside the package in case of damage to the outside copy. However, this may vary from country to country. For example, regulations in the United States specify that all documents should be inside the package.
-
A copy of all documents sent with the consignments should be retained by the collector.
Preparation of samples for pest identification
Arrangements should be made in advance of the fieldwork with the institutes that are to receive samples for pest identification. Permits may have to be obtained to comply with the quarantine requirements of the country where samples are to be sent. Some additional points:
-
All material sent for identification purposes, whether preserved insects and mites, dried plant voucher specimens of diseased plants or living plant material for diagnosis of internal pathogens, should be labelled
-
with a reference number, as well as
-
the botanical name of the host plant
-
the locality where collected
-
the date of collecting
-
the name of the collector(s)
-
-
Collectors should keep a copy of the information accompanying each specimen.
-
Samples of seeds and pollen may have to be sent for viability testing, as well as for inspection for internally-borne pathogens, and weeds. Samples should be properly dried before dispatch.
-
Collectors should include the name of the person (and address) to whom the identification(s) should be sent.
Future challenges/needs/gaps
And what of the future? The challenge is for samples to move ever faster, and ever more safely, between countries, from places of collection to places of storage and other use. This requires collectors and quarantine officials to work together in more consistent and coherent ways – in itself a major challenge.
Collectors and the users of plant germplasm would like quarantine officials to act faster. Quarantine officials (ever mindful of their mandate to protect crops and indigenous flora from new pest incursions) need time if they are to do their job thoroughly. Tensions can arise from these, seemingly, different ways of viewing germplasm, but this is a false dichotomy. All sides have to work together if satisfactory outcomes are to be achieved: the rapid international movement of germplasm safe from risk.
If this is not achieved, there will be – or continue to be – adverse consequences. We know from anecdotal evidence that long delays in quarantine bring frustrations and, unfortunately, a temptation among some to short-circuit official systems, especially where these are not well developed. It is the responsibility of those sponsoring collecting missions to forbid such practices.
So are there ways to reduce the time it takes to process samples, check for associated pests? Some suggestions are obvious: crop-specific guidelines for testing germplasm for the pests of concern need to be produced, and they need to be updated constantly. Those produced by Bioversity International (also under its previous names as IBPGR and IPGRI) are expensive to produce and become outdated too soon. This needs to change. Documents need to be posted on the internet, and frequent revisions made as new data comes to hand. The linking of institutes – international and national – dealing with indexing technologies of specific crops needs to continue. And conformity of national quarantine regulations needs to be achieved. There are still countries that re-index samples, no matter that they have been indexed elsewhere, causing considerable frustration to would-be users.
Another question is whether molecular technologies can assist in speeding up the processing of germplasm samples. The most likely answer is both a “yes” and a “no”. Several current programmes that “fingerprint” and “bar-code” plants and animals will, undoubtedly, lead to speedy identifications of pests attached to germplasm being moved internationally. It may also speed up the process of determining whether pathogens are present within plant tissues.
The problem, however, is what to do about the detection of DNA within a plant that differs from that of the plant itself, particularly if there are no symptoms of any kind. Currently, plants that are symptomless after months or years (most often a crop cycle) in quarantine are released to the importer. In future, national phytosanitary officials may have to deal with the quandary of what to do about symptomless plants containing apparently alien DNA. There is no guarantee that once released into a new environment the endophytic organism will not be transferred to another plant (perhaps by an insect vector) and become a pest. Decision making in this area is set to become extremely difficult.
As mentioned already in this chapter, the internet has grown greatly over the years since this book was first published, and is now of immense value to researchers and national phytosanitary officials alike. They now have almost instant access to all the knowledge available on a particular organism, much of it being kept up to date. Perhaps the next phase, already beginning in some countries, will be to enable researchers, industry and governments to collaborate, use expertise, share data and information, and generate intelligence through the development of information technologies akin to the popular social networks. An example of this is the web portal of the Australian Biosecurity Intelligence Network (ABIN): www.abin.org.au/web/index.html.
Development of biosecurity networks will be a challenge within countries where researchers may guard certain information prior to publication, but it will be even more challenging between countries where the trade implications of the presence or absence of a pest can have huge economic consequences. Nevertheless, the benefits of information sharing are already self-evident following the growth of the internet, and it is to be hoped that attempts at greater information sharing through networking will be equally positive.
Conclusions
Countries throughout the world are keen to safeguard agriculture, forestry and the environment from potentially threatening invasive pest species. This commitment is regulated under the International Plant Protection Convention with support, in recent years, from the World Trade Organization’s Agreement on the Application of Sanitary and Phytosanitary Measures (the SPS Agreement). Governments apply measures for food safety and animal and plant health – sanitary and phytosanitary measures – to impede the spread of pests. Collectors of germplasm must be aware of these developments, and the potential harm that the unrestricted movement of germplasm could cause. They must ensure that samples conform to standards set under both national legislation and international regulations. Failure to do this could jeopardize collecting missions.
Back to list of chapters on collecting
References and further reading
General
AAB. 1985 et seq. Descriptions of Plant Viruses. Association of Applied Biologists, Wellesbourne, UK.
Altieri MA. 1993. Ethnoscience and biodiversity: key elements in the design of sustainable pest management systems for small farmers in developing countries. Agriculture, Ecosystems and Environment 46:257–272.
Bland RG, Jacques HE. 1978. How To Know Insects. WC Brown Company Publishers, Dubuque, Iowa.
Borror DJ, Delong DM, Triplehorn CA. 1976. An Introduction to the Study of Insects. Holt, Rinehart and Winston, New York.
British Museum (Natural History). 1974. Insects. Instructions for collectors No.4a. British Museum (Natural History), London.
Brunt AA, Crabtree K, Gibbs A. 1990. Viruses of Tropical Plants. CAB International, Slough, UK.
Brunt AA, Crabtree K, Dallwitz M, Gibbs A, Watson L, Zurcher E, editors. 1996. Plant Viruses Online: Descriptions and Lists from the VIDE Database. CAB International, Wallingford, UK. Available online (accessed 15 August 2011): www.agls.uidaho.edu/ebi/vdie/refs.htm.
CMI/AAB. 1970-1984. Descriptions of Plant Viruses. Sets 1–18. CAB International and Association of Applied Biologists, Slough and Wellesbourne, UK.
FAO. 1993. Directory of Regional Plant Protection Organizations and National Plant Quarantine Services. AGPP/Misc /93/1. FAO, Rome.
FAO. 2011. International Standards for Phytosanitary Certificates. ISPM 12. FAO, Rome. Available online (accessed 15 August 2011): https://www.ippc.int/file_uploaded/1307528241_ISPM_12_2011_En_2011-05-03(Corre.pdf.
Hall GS, Hawksworth DL. 1990. International Mycological Directory. CAB International, Wallingford, UK.
Hewitt WB, Chiarappa L. 1977. Plant Health and Quarantine in International Transfer of Genetic Resources. CRC, Cleveland, Ohio.
IIE. 1968 et seq. Distribution Maps of Pests. Series A (Agriculture). International Institute of Entomology (formerly Commonwealth Institute of Entomology) and CAB International, Wallingford, UK.
IIP. 1972 et seq. IIP Descriptions of Plant-Parasitic Nematodes. International Institute of Parasitology (formerly Commonwealth Institute of Helminthology) and CAB International, Wallingford, UK.
IMI. 1942 et seq. IMI Distribution Maps of Plant Diseases. International Mycological Institute (formerly Commonwealth Mycological Institute) and CAB International, Wallingford, UK.
IMI. 1964 et seq. IMl Descriptions of Fungi and Bacteria. International Mycological Institute (formerly Commonwealth Mycological Institute) and CAB International, Wallingford, UK.
IPPC. 2010. Glossary of Phytosanitary Terms. ISPM No. 5. International Plant Protection Convention, Rome. Available online (accessed 15 August 2011): https://www.ippc.int/file_uploaded/1273490046_ISPM_05_2010_E.pdf.
Johnston A, Booth C. 1983. Plant Pathologist's Pocketbook. CAB International, Wallingford, UK.
Nelson SC, Bushe BC. 2006. Collecting Plant Disease and Insect Pest Samples for Problem Diagnosis. SCM-14. Cooperative Extension Service, University of Hawaii at Manoa, Honolulu, Hawaii. Available online (accessed 29 August 2011): www.ctahr.hawaii.edu/oc/freepubs/pdf/SCM-14.pdf.
Sonoda RM. 1979. Collection and preservation of insects and pathogenic organisms. In: Mott GO, Jimenez A, editors. Handbook for the Collection, Preservation and Characterization of Tropical Forage Germplasm Resources. CIAT, Cali, Colombia.
Waller JM, Lenné JM, Waller S. 2001. Plant Pathologists’ Pocketbook. 3rd Edition. CAB International, Wallingford, UK.
American Phytopathological Society online bookshop: www.cplbookshop.com/glossary/G583.htm
Australian Biosecurity Intelligence Network (ABIN): www.abin.org.au/web/index.html
Bioversity International descriptor lists: www.bioversityinternational.org/research/conservation/sharing_information/descriptor_lists.html
CABI Crop Protection Compendium (CPC): www.cabi.org/cpc
Crop Genebank Knowledge Base: http://cropgenebank.sgrp.cgiar.orgindex.php?option=com_content&view=article&id=137&Itemid=238&lang=english
Descriptions of fungi and bacteria (IMI): www.cabi.org/default.aspx?site=170&page=1016&pid=2214
Descriptions of plant viruses (CMIIAAB): www.dpvweb.net
Distribution maps of pests (lIE): www.cabi.org/default.aspx?site=170&page=1016&pid=2212
Distribution maps of plant diseases (IMI): www.cabi.org/default.aspx?site=170&page=1016&pid=2210
FAO/IPPC list of plant-protection services worldwide: https://www.ippc.int/index.php?id=1110520&no_cache=1&type=contactpoints&L=0
Global Plant Clinic: www.cabi.org/default.aspx?site=170&page=1017&pid=2301
International Plant Protection Convention (lPPC): https://www.ippc.int/index.php?id=1110589&L=0
Natural History Museum, South Kensington, UK: www.nhm.ac.uk/about-us/contact-enquiries/identification-and-general-science-enquiries/index.html
Examples of useful databases on plants and pests
|
Arthropods of Economic Importance: Agromyzidae |
|
|
Arthropods of Economic Importance: Diaspididae |
http://nlbif.eti.uva.nl/bis/diaspididae.php?menuentry=inleiding |
|
Australian Faunal Director |
www.environment.gov.au/biodiversity/abrs/online-resources/fauna/afd/home |
|
Databases and Publications from Kew |
www.kew.org/science-research-data/databases-publications/index.htm |
|
Descriptions of Plant Viruses |
|
|
Diseases and disorders of cultivated palms |
|
|
EcoPort |
|
|
EPPO Database on Diagnostic Expertise |
|
|
Fauna Europea |
|
|
Flora of New Zealand |
|
|
ICTVdB Index of Viruses |
|
|
Lucidcentral Key Search |
www.lucidcentral.com/Keys173/SearchforaKey/tabid/217/language/en-US/Default.aspx |
|
Index Fungorum |
|
|
Mansfeld's World Database of Agricultural and Horticultural Crops |
http://mansfeld.ipk-gatersleben.de/pls/htmldb_pgrc/f?p=185:3:4166990628405234 |
|
NLBIF Biodiversity Data Portal |
|
|
Landcare Research (New Zealand) |
|
|
Pacific Islands Pest List Database |
|
|
Plant Viruses Online |
|
|
The Global Lepidoptera Names Index |
www.nhm.ac.uk/research-curation/research/projects/lepindex/index.html |
|
USDA ARS Fungal Databases |
|
|
USDA ARS Germplasm Resources Information Network (GRIN) Taxonomy for Plants |
|
|
USDA ARS Systematic Entomology Laboratory |
|
|
Wikispecies |
FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm Series
Acacia spp.
Old KM, Vercoe TK, Floyd RB, Wingfield MJ, Roux J, Neser S, editors. 2002. Acacia spp. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm No. 20. FAO/IPGRI, Rome.
Allium spp.
Diekmann M. 1997. FAO/IPGRI Technical Guidelines for the Safe Movement of Allium spp. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm No. 18. FAO/IPGRI, Rome.
Cacao
Frison EA, Diekmann M, Nowell D, editors. 2000. FAO/IPGRI Technical Guidelines for the Safe Movement of Cocoa Germplasm. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm No. 20. FAO/IPGRI, Rome.
Cassava
Frison EA, Feliu E, editors. 1991. FAO/IBPGR Technical Guidelines for the Safe Movement of Cassava Germplasm. FAO/IBPGR, Rome.
Citrus
Frison EA, Taber M, editors. 1991. FAO/IBPGR Technical Guidelines for the Safe Movement of Citrus Germplasm. FAO/IBPGR, Rome.
Coconut
Frison EA, Putter CAJ, Diekmann M, editors. 1993. FAO/IBPGR Technical Guidelines for the Safe Movement of Coconut Germplasm. FAO/IBPGR, Rome.
Edible aroid
Zettler FW, Jackson GVH, Frison EA. 1989 FAO/IBPGR Technical Guidelines for the Safe Movement of Edible Aroid Germplasm. FAO/IBPGR, Rome.
Eucalyptus spp
Ciesla WM, Diekmann M, Putter CAJ, editors. 1996. Eucalyptus spp. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm No. 17. FAO/IPGRI, ACIAR & ASEAN, Rome.
Grapevine
Frison EA, lkin R, editors. 1991. FAO/IBPGR Technical Guidelines for the Safe Movement of Grapevine Germplasm. FAO/IBPGR, Rome.
Legumes
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD, editors. 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. FAO/IBPGR, Rome.
Musa spp.
Diekmann M, Putter CAJ. 1996. FAO/IPGRI Technical Guidelines for the Safe Movement of Musa Germplasm. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm No. 15. 2nd Edition. FAO/IPGRI, Rome.
Pinus spp.
Diekmann M, Sutherland JR, Nowell DC, Morales FJ, Allard G, editors. 2002. Pinus spp. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm No. 21. FAO/IPGRI, Rome.
Potato (Solanum tuberosum)
Jeffries C J. 1998. Potato. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm No. 19. FAO/IPGRI, Rome.
Small fruits
Diekmann M, Frison EA, Putter CAJ, editors. 1994. FAO/IPGRI Technical Guidelines for the Safe Movement of Small Fruit Germplasm. FAO/IPGRI, Rome.
Small grain temperate cereals
Diekmann M, Putter CAJ, editors. 1995. Small Grain Temperate Cereals. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm No. 14. FAO/IPGRI, Rome.
Stone fruits
Diekmann M, Putter CAJ, editors. 1994. FAO/IPGRI Technical Guidelines for the Safe Movement of Stone Fruit Germplasm. FAO / IPGRI Technical Guidelines for the Safe Movement of Germplasm No. 16. FAO/IPGRI, Rome.
Sugarcane
Frison EA, Putter CAJ, editors. 1993. FAO/IBPGR Technical Guidelines for the Safe Movement of Sugarcane Germplasm. FAO/IBPGR, Rome.
Sweet potato (Ipomoea batatas)
Moyer JW, Jackson GVH, Frison EA. 1989. FAO/IBPGR Technical Guidelines for the Safe Movement of Sweet Potato Germplasm. FAO/IBPGR, Rome.
Vanilla
Pearson MN, Jackson GVH, Zettler FW, Frison EA. 1991. FAO/IBPGR Technical Guidelines for the Safe Movement of Vanilla Germplasm. FAO/IBPGR, Rome.
Yam (Dioscorea spp.)
Brunt AA, Jackson GVH, Frison EA. 1989. FAO/IBPGR Technical Guidelines for the Safe Movement of Yam Germplasm. FAO/IBPGR, Rome.
The American Phytopathological Society (APS) publications on the identification of plant diseases (www.apsnet.org/publications/Pages/default.aspx)
|
Compendium of Corn Diseases (Third Edition) |
Shade Tree Wilt Diseases |
Appendix 17.1: IPPC Model Phytosanitary Certificate
(Download IPPC Model Phytosanitary Certificate (0.9 MB) for printing purposes)
 |
|
Source: FAO (2011) |
Chapter 25: Collecting pollen for genetic resources conservation
Gayle M. Volk
National Center for Genetic Resources Preservation USDA-ARS, Fort Collins, USA
E-mail: Gayle.Volk(at)ars.usda.gov
|
2011 version |
1995 version |
||
|
Open the full chapter in PDF format by clicking on the icon above. |
|||
This chapter is a synthesis of new knowledge, procedures, best practices and references for collecting plant diversity since the publication of the 1995 volume Collecting Plant Genetic Diversity: Technical Guidelines, edited by Luigi Guarino, V. Ramanatha Rao and Robert Reid, and published by CAB International on behalf of the International Plant Genetic Resources Institute (IPGRI) (now Bioversity International), the Food and Agriculture Organization of the United Nations (FAO), the World Conservation Union (IUCN) and the United Nations Environment Programme (UNEP). The original text for Chapter 25: Collecting Pollen for Genetic Resources Conservation, authored by F. A. Hoekstra, has been made available online courtesy of CABI. The 2011 update of the Technical Guidelines, edited by L. Guarino, V. Ramanatha Rao and E. Goldberg, has been made available courtesy of Bioversity International.
Please send any comments on this chapter using the Comments feature at the bottom of this page. If you wish to contribute new content or references on the subject please do so here.
Back to list of chapters on collecting
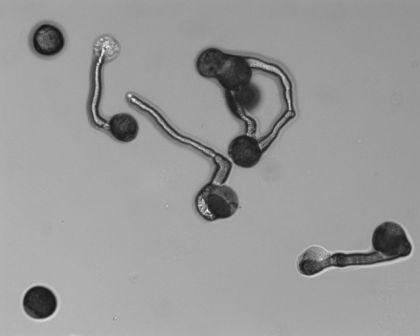 |
|
|
Germinating pecan pollen. (Photo: J. Waddell/USDA-ARS - National Center for Genetic Resources Preservation). |
Abstract
Pollen is a useful source of diverse alleles within a genepool and so may be an effective propagule for genebanks. The ease of pollen storage and shipment and the potential for its immediate use provide researchers with increased options when designing their breeding programs. Methods for pollen collection, desiccation, viability testing and longevity assessment have been developed for many species of interest, and have revealed the critical importance for increased longevity by using high quality pollen desiccating it sufficiently in a rapid manner and subsequently storing it at very low temperatures. Reliable viability assessments are dependent upon adequate rehydration and the use of reliable stains, in vitro germination assays or in vivo pollination experiments. Pollen preservation in genebanks will likely be implemented as standard procedures for handling and assessing it are developed.
Current Status
Advantages to the use of pollen
-
Genebanked pollen can be made available to breeders upon request. For tree species, this obviates the need for growing the male parents in the breeding orchards. It allows for wide hybridization across seasonal and geographical limitations, and reduces the coordination required to synchronize flowering and pollen availability for use in crosses (Bajaj 1987). With adequate pollen available, one can also load additional pollen onto stigmas to increase pollination and yield.
-
Pollen is available for research programs. As single cells, pollen provides a simple model system for research on conservation. Storage of pollen within genebanks also ensures its availability year-round for basic biology and allergy research programs (Shivanna 2003).
-
Pollen captures diversity within small sample sizes, and documentation is available for long-term survival of pollen from many diverse species (table 25.1). Pollen also serves as a source of genetic diversity in collections where it is hard to maintain diversity with seeds (species of low fecundity, large seeds, or seeds that require an investment of labour to store).
-
Pollen can also be shipped internationally, often without threat of disease transfer (Hoekstra 1995).
Table 25.1. A Selection of Species for Which Pollen Can Be Successfully Stored at –80°C or Liquid Nitrogen (LN) Temperatures
|
Species |
Storage duration |
Temperature |
Viability test |
Reference |
|
Actinidia |
1 y |
LN |
in vitro germ, pollination |
Abreu and Oliveira 2004 |
|
Aechmea |
15 min |
LN |
in vitro germ |
Parton et al. 2002; Parton et al. 1998 |
|
Allium |
1 y |
LN |
pollination |
Ganeshan 1986b |
|
Beta |
17 y |
LN |
FDA, MTT, pollination |
Panella et al. 2009 |
|
Beta |
1 y |
LN |
pollination |
Hecker et al. 1986 |
|
Carica |
485 d |
LN |
in vitro germ, pollination |
Ganeshan 1986a |
|
Carya |
13 y |
LN |
in vitro germ |
Sparks and Yates 2002 |
|
Carya |
1 y |
LN |
pollination |
Yates and Sparks 1990 |
|
Carya |
3 y |
-80 |
in vitro germ, pollination |
Yates and Sparks 1990 |
|
Citrus |
3.5 y |
LN |
in vitro germ, pollination |
Ganeshan and Alexander 1991 |
|
Clianthus |
3 h |
LN |
in vitro germ |
Hughes et al. 1991. |
|
Dioscorea |
2 y |
-80 |
acetocarmine |
Ng and Daniel 2000 |
|
Elaeis |
8 y |
LN |
FDA, in vitro germ |
Tandon et al. 2007 |
|
Gladiolus |
10 y |
LN |
in vitro germ, pollination |
Rajasekharan et al. 1994 |
|
Glycine |
7 d |
LN |
pollination |
Tyagi and Hymowitz 2003 |
|
Guzmania |
15 min |
LN |
in vitro germ, pollination |
Parton et al. 2002 |
|
Humulus |
2 y |
LN |
pollination |
Haunold and Stanwood 1985 |
|
Juglans |
2 y |
LN |
in vitro germ |
Farmer and Barnett 1974 |
|
Juglans |
1 y |
LN |
in vitro germ, pollination |
Luza and Polito 1987 |
|
Lycopersicon |
5 wk |
-80 |
pollination |
Sacks and St. Clair 1996 |
|
Lycopersicon |
22 mo |
LN |
in vitro germ, pollination |
Karipidis et al. 2007 |
|
Olea |
1 h |
LN |
in vitro germ |
Parfitt and Almehdi 1984a |
|
Panax |
11 mo |
LN |
stain, in vitro germ, pollination |
Zhang et al. 1993 |
|
Persea |
1 y |
LN |
pollination |
Sedgley 1981 |
|
Phoenix |
435 d |
LN |
in vitro germ, pollination |
Tisserat et al 1983 |
|
Protea |
1 y |
LN |
in vitro germ, pollination |
Van der Walt and Littlejohn 1996 |
|
Prunus |
12 mo |
-80 |
in vitro germ |
Martinez-Gómez et al. 2002 |
|
Prunus |
1 h |
LN |
in vitro germ |
Parfitt and Almehdi 1984b |
|
Pyrus |
3 y |
LN |
pollination |
Akihama and Omura 1986 |
|
Rosa |
8 wk |
LN |
in vitro germ |
Marchant et al. 1993 |
|
Rosa |
1 y |
LN |
hanging drop, fertilization |
Rajasekharan and Ganeshan 1994 |
|
Solanum |
10 min |
LN |
in vitro germ |
Towill 1981 |
|
Tillandsia |
15 min |
LN |
in vitro germ |
Parton et al. 2002 |
|
Vitis |
64 wk |
LN |
in vitro germ |
Ganeshan 1985a |
|
Vitis |
5 y |
LN |
in vitro germ, pollination |
Ganeshan and Alexander 1990 |
|
Vitis |
1 h |
LN |
in vitro germ |
Parfitt and Almehdi 1983 |
|
Vriesea |
15 min |
LN |
in vitro germ |
Parton et al. 2002 |
|
Zea |
120 d |
LN |
in vitro germ, pollination |
Barnabás and Rajki 1976 |
Disadvantages to the use of pollen
-
Limited pollen production in some species. The primary limitation in the routine implementation of pollen storage within genebanks is the difficulty in obtaining adequate quantities of pollen for many species.
-
Labour-intensive collection or processing. For some species, pollen is readily available, but resources to accumulate and process enough pollen for routine storage and distribution are inadequate.
-
No standardized processing or viability-testing protocols. Processing and viability-testing methods have not been documented and standardized in a manner similar to that of seed testing.
-
Regeneration of aged pollen. Seed regeneration can often be performed directly using the seed samples in storage. For pollen, associated mother plants are necessary to replenish pollen supplies when quantities are depleted or have deteriorated (Schoenike and Bey 1981).
Pollen collection
Collected pollen serves to maintain and preserve the alleles of an individual or population. Sampling strategies have often recommended collecting a set number of individuals per population to ensure that the common alleles are captured. The exact number of individuals that most effectively captures the genetic variation is dependent upon the genetic diversity and life-history traits of the species (Lockwood et al. 2006). Namkoong (1981) suggests that collecting pollen from a single tree easily captures the alleles for that genotype; however, it is recommended that a minimum of 68 trees be sampled to represent a wild population. Pollen can also be collected from individual trees within a genebank both to conserve alleles specific to each individual and to provide male gametes for breeding purposes. Although only small quantities of pollen are required to capture the genes of an individual, because of the challenges of pollen collection and processing, it might be more efficient to collect larger quantities to ensure its long-term availability to the user community.
Pollen should be harvested soon after anthesis, usually in the morning hours (Ganeshan et al. 2008; Towill 2004). Shelf life is short for pollen collected from immature, aged, or weather-damaged anthers (Towill 1985). It is usually more practical to collect anthers in the field and then separate the pollen grains from the anthers in a laboratory environment soon after collection. All pollen must be processed immediately (within hours) to ensure maximum potential longevity.
 |
|
|
Storage of pollen for long-term preservation |
Pollen desiccation
Successful pollen genebanking is dependent upon achieving long-term survival of stored pollen. Water content, cooling rate and storage temperature all affect the longevity of stored pollen (Buitink et al. 1996, 2000). Field conditions and relative humidity at the time of harvest affect the pollen moisture content, and germinability is impaired when pollen is kept for any length of time in wet or high-humidity conditions (Hoekstra 1986). Pollen ages quickly when held at 24°C and 75% relative humidity (RH) (Van Bilsen et al. 1994).
For desiccation-tolerant pollen, it is critical that the pollen be dried to a target moisture content soon after harvest. Depending on species, successful long-term storage requires that the moisture content be reduced to or below levels at which there is no free water (Priestley 1986). For many species, pollen can be dried to water contents of 0.05 g H2O g-1 dry weight (DW) without a loss in viability (Hoekstra 1986). This can be achieved by drying overnight in a low-humidity room environment or over salt chambers that are maintained at RH of about 30%. Equilibration over salt slurries, such as magnesium chloride or calcium nitrate, prevents damage that could result from over drying within ovens. It is a straightforward method to control moisture content in diverse laboratory environments (Connor and Towill 1993; Towill 1985).
Anthers or pollen grains can also be dried over silica gel at room temperature (Ganeshan 1985; Parfitt and Ganeshan 1989; Parton et al. 1998; Sacks and St. Clair 1996; Van der Walt and Littlejohn 1996). Martinez-Gómez et al. (2002) successfully desiccated almond pollen with silica gel for 48 hours at 22°C for long-term storage. Sato et al. (1998) dehydrated anthers at 20°C for 16–24 hours at RH of 15% or 32% prior to storage. Although some researchers have demonstrated successful desiccation through the use of freeze-driers for pollen desiccation, concerns have arisen with regard to maintaining viability in pollen that has been frozen prior to dehydration (Ganeshan and Alexander 1986, 1987; Perveen and Khan 2008). Towill (1985) argued that vacuum drying was as effective as freeze-drying for maintaining pollen viability. Although air at low RH will increase the drying rate, pollen must be removed before it dries to a lethal moisture level (1% to 2 % for peach and pine, 3.5% for coconut pollen) (Towill 1985). Pollen can be successfully dried in 35°C ovens, but care must be taken not to over dry under these conditions (Yates et al. 1991).
Rapid air-drying can also be achieved by using specialized pollen-dryers that blow air at 20% to 40% RH and 20°C, to quickly reduce moisture content in the pollen of Poaceae species, including Avena, Pennisetum, Saccharum, Secale, Triticum, Tricosecale and Zea (Barnabás and Kovacs 1996). Maize pollen is easily stored when quickly dried to 0.19 g H2O g-1 DW (Buitink et al. 1996).The longevity of the pollen from these desiccation-sensitive species, and its tolerance to freezing temperatures, has been extended as a result of using rapid dehydration methods. The principle of rapid drying (flash drying) has successfully been documented in recalcitrant seeds, where it was shown that one could dry to a much lower water content if one did it rapidly (Pammenter et al. 1991).
Storage temperature
It is possible to store pollen of many species at temperatures between 4°C and –20°C for the short-term. Dry pollen that is kept at between 4°C and –20°C remains viable for a few days to a year, which may be adequate for use in breeding programs (Hanna and Towill 1995).
Long-term viability can be maintained by storing pollen at –80°C or LN temperatures (–196°C) (Hanna and Towill 1995). Once desiccated, pollen can be dispensed into cryovials for long-term storage in LN or LN vapour. Precise labelling of vials and storage locations is recommended to aid in future retrieval of samples. Vials can then be placed in boxes or cryocanes and directly immersed in the liquid or vapour phase of liquid nitrogen (Barnabás and Kovács 1996; Ganeshan et al. 2008; Hanna and Towill 1995; Connor and Towill 1993).
Pollen rehydration
Dried pollen is susceptible to injury from rapid water update during rehydration (also known as imbibitional injury) (Hoekstra and Van der Wal 1988), which can severely reduce germination and lead to low viability counts if vital staining (stains to identify living cells) is used to assess it. Low temperatures can exacerbate imbibitional damage, which is believed to arise from mechanical damage to the plasma lemma as polar lipids undergo phase changes as a result of fluctuations in temperature, water content and sugars (Hoekstra et al. 1992; Hoekstra and Van der Wal 1988; Crowe et al. 1989). Slow rehydration ameliorates imbibitional damage to pollen grains and this is usually accomplished by placing the pollen in a humid environment prior to direct liquid exposure (Hoekstra and Van der Wal 1988; Luza and Polito 1987; Parton et al. 2002). Pollen rehydration can be as straightforward as placing open vials of pollen in 100% humidity environments for 1 to 4 hours at room temperature (Connor and Towill 1993; Hanna and Towill 1995).
Although suboptimal storage conditions may affect pollen vigour before a measurable change in pollen viability is observed, most studies make use of viability assessments (Shivanna et al. 1991). Pollen viability can be measured by vital staining pollen grains, by germinating pollen grains in vitro, or by demonstrating successful fertilization and seed development in plants.
Pollen viability
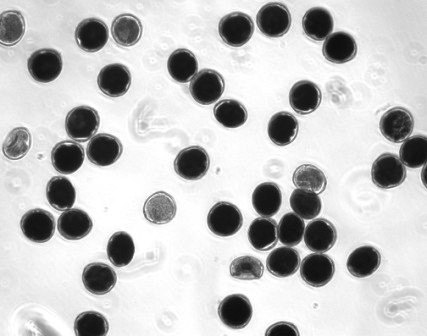 |
|
|
Viability staining (thiazolyl blue tetrazolium) for pecan pollen grains (photo: John Waddell, USDA-ARS-National Center for Genetic Resources Preservation). |
Staining
One commonly used vital stain is the fluoregenic ester, fluorescein diacetate (FDA). This test measures membrane integrity. Pollen grains fluoresce green when a cellular esterase cleaves the FDA (Heslop-Harrison and Heslop-Harrison 1970). Since this assay is dependent upon functional membranes, the osmoticum of the FDA staining solution is critical; stain is often dissolved in a 10% to 20% sucrose solution containing boric acid and calcium nitrate to minimize plasmolysis and membrane leakage.
Comparisons of viability determined through the use of FDA or tetrazolium and those obtained using in vitro germination or in vivo fertilization tests reveal consistently high correlations, provided pollen is adequately rehydrated prior to testing (Firmage and Dafni 2001; Khatun and Flowers 1995; Rodriguez-Riano and Dafni 2000; Shivanna and Heslop-Harrison 1981). FDA has occasionally been shown to give false negative results, where viable pollen appears dead (Heslop-Harrison et al. 1984).
Several tetrazolium-based stains are available for testing pollen viability (Norton 1966). The 3 (4,5-dimethyl thiazolyl 1-2) 2,5-diphenyl tetrazolium bromide (MTT) test was shown to give the most dependable results in a comparison trial using plum pollen (Norton 1966). In general, tetrazolium tests measure the ability to reduce colourless tetrazolium to coloured formazan, thus identifying pollen that has a capacity for oxidative metabolism (Hauser and Morrison 1964).
Many other vital stains have been developed and proposed over the past 50 years. Stains such as Alexander‟s, acetocarmine, aniline blue and X-gal have been shown to be successful identifiers of viability for relatively few species or under specialized conditions (Rodriguez-Riano and Dafni 2000). Viability results obtained with these stains may not correlate well with in vitro germination assays (Towill 1985).
In vitro germination
Pollen can be germinated in vitro by placing pollen grains onto a medium and measuring the elongation of the pollen tube after a few hours of imbibition. Pollen tubes that elongate to a length that is at least the diameter of the pollen grain are considered viable (Dafni and Firmage 2000). Automated counting procedures using morphometry software result in pollen counts that are within 5% of visual observations and allow the determination of pollen tube length in addition to the data obtained by eye on tube presence or absence (Pline et al. 2002). These automated systems may expedite time-consuming assays of in vitro pollen germination. As for viability testing, it is important to implement repeatable and standardized methods and to use dead pollen samples as controls.
The optimal temperature for in vitro germination assays can be species dependent. The pollen from many species germinates well at 25°C; however, differences exist. For example, cotton pollen has an optimum germination temperature of 28°C to 31°C (Burke et al. 2004). Hence, for the purpose of pollen conservation, such information should be known for the target species.
In vitro germination methods utilize pollen immersed in aerated solutions, “hanging” drops, or dispersed on solidified medium. The medium is often that described by Brewbacker and Kwack (1963) or a slight modification thereof. Boric acid, calcium nitrate and sucrose concentrations in the medium might have to be optimized according to species (Bolat and Pirlak 1999; Heslop-Harrison 1992). The hanging-drop method involves the placement of a slide or coverslip with liquid medium and pollen inverted over a 100% humidity chamber (Rajasekharan and Ganeshan 1994). For observation, the slide is returned to an upright position and observed under a microscope.
Pollination
Testing viability by observing pollen tube elongation within the stigma or fertilization and subsequent seed production is the most time-intensive way to determine pollen viability; however, these kinds of tests are also the most relevant to demonstrate the adequacy of the pollen for use. Rehydrated pollen can be placed on the stigmas of live plants, and tube length is measured after a pre-determined time interval (Dafni and Firmage 2000). Ideally, successful fruit set and seed production occurs after pollination with conserved pollen. Marquard (1992) demonstrated that high levels of viability are not required. Fruit set occurred when stigmas were treated with only 5% viable pollen. When pollen appears viable based on germination tests, it is often also viable in fertilization assays.
Pollen longevity
The longevity of pollen is dependent upon many factors specific to cultivars or species as well as handling procedures, as discussed here (Ganeshan and Alexander 1991; Hanna and Towill 1995). The presence of sucrose and polysaccharides in the pollen has been correlated with protection of membranes from desiccation or temperature stress and may confer greater longevity (Dafni and Firmage 2000; Hoekstra et al. 1989). High-quality pollen dehydrated to an optimal moisture content and stored at LN temperatures has been documented to store for well over 10 years (Panella et al. 2009; Sparks and Yates 2002). The low temperature reduces the molecular mobility in the cytoplasm, which may be a controlling factor in pollen longevity (Buitink et al. 2000). The aging of dried pollen is likely caused by oxidative reactions, and pollens with higher levels of unsaturated fatty acids usually have a shorter shelf life (Hoekstra 2005). Correspondingly, pollen longevity may be further improved by storing desiccated pollen in an oxygen-free atmosphere (Hoekstra 1992).
Most reports describing pollen survival after LN exposure state viability levels after determined lengths of time, often without initial germination data. These end-point levels serve to demonstrate that the tested length of storage is possible, but they do not describe the longevity of the pollen per se. Table 25.1 demonstrates the diverse range of species for which pollen can be placed at LN temperatures. According to current information, it is clear that both desiccation-tolerant and non-desiccation-tolerant pollen types can be stored for over 10 years under controlled conditions (Barnabás1994; Barnabás and Kovács 1996; Shivanna 2003). Thus, despite additional challenges that may be present in storing desiccation-sensitive pollen, it is possible. Additional research is needed to determine how long both types of pollen will remain viable under these conditions.
Future challenges/needs/gaps
Technologies for successful pollen conservation have been developed and are available. A set of standardized methods to process pollen types with different physiologies is needed to make pollen storage a routine effort in genebanks. For many species in need of pollen conservation, we need to know more about the phenology of pollen production so that we can properly time pollen harvests. Standards should be developed for pollen collection, processing and storage of desiccation-tolerant and non-desiccation-tolerant pollen types. Determination of pollen genebanking standards is an initial step towards implementing pollen genebanking methods.
The literature currently lists the age and viability of pollen from many species stored at LN temperatures (table 25.1) (Barnabás and Kovács 1996; Ganeshan and Rajashekaran 2000; Hanna and Towill 1995; Towill 1985). However, the viability over time, or longevity, of pollen stored under LN genebanking conditions has not been thoroughly evaluated. In addition, detailed biophysical studies should be pursued to determine the optimal water content, desiccation rates and longevity relationships for various pollen types. Longevity must be known in order to ascertain the cost and benefits of genebanking pollen.
Conclusions
There are abundant reports in the literature of many successes for testing pollen viability and temperature exposure. Many of the reported data and methods are difficult to replicate when basic parameters such as initial water content, equilibrated (desiccated) water content and rehydration methods are not described (Dafni and Firmage 2000). It is clear that the physiological state of the pollen at the time of collection and the handling of that pollen within the first few days after collection will determine its potential for long-term survival under optimum conditions. Detailed reporting of handling upon harvest is essential if standardized methods are to be developed. Confirmation that reported staining, in vitro germination and in vivo germination results are correlated increases confidence in and the repeatability of the reported data.
Despite the challenges, pollen is a valuable genetic resource for conservation. It provides breeders and researchers with an additional, complementary, propagule that may be immediately useful in their programs, although the feasibility of pollen collection and preservation varies among plant species.
Back to list of chapters on collecting
References and further reading
Abreu I, Oliveira M. 2004. Fruit production in kiwifruit (Actinidia deliciosa) using preserved pollen. Australian Journal of Agricultural Research 55:565–569.
Akihama T, Omura M. 1986. Preservation of fruit tree pollen. In: Bajaj YPS, editor. Biotechnology in Agriculture and Forestry. Vol. 1: Trees. Springer Verlag, Berlin. pp.101–112.
Bajaj YPS. 1987. Cryopreservation of pollen and pollen embryos, and the establishment of pollen banks. International Review of Cytology 107:397–420.
Barnabás B. 1994. Preservation of maize pollen. In: Bajaj YPS, editor. Biotechnology in Agriculture and Forestry. Vol. 25. Springer Verlag, Berlin. pp. 608–618.
Barnabás B, Kovács G. 1996. Storage of pollen. In: Shivanna KR, Sawhney VK, editors. Pollen Biotechnology for Crop Production and Improvement. Cambridge University Press, Cambridge, UK. pp. 293–314.
Barnabás B, Rajki E. 1976. Storage of maize (Zea mays L.) pollen at -196oC in liquid nitrogen. Euphytica 25:747–752.
Bolat I, Pirlak L. 1999. An investigation on pollen viability, germination and tube growth in some stone fruits. Turkish Journal of Agriculture and Forestry 23:383–388.
Brewbacker JL, Kwack BH. 1963. The essential role of calcium ion in pollen germination and pollen tube growth. American Journal of Botany 50:859.
Buitink J, Leprince O, Hemminga MA, Hoekstra FA. 2000. The effects of moisture and temperature on the ageing kinetics of pollen: interpretation based on cytoplasmic mobility. Plant, Cell and Environment 23:967–974.
Buitink J, Walters-Vertucci C, Hoekstra FA, Leprince O. 1996. Calorimetric properties of dehydrating pollen. Plant Physiology 111:235–242.
Burke JJ, Velten J, Oliver MJ. 2004. In vitro analysis of cotton pollen germination. Agronomy Journal 96:359–368.
Connor KF, Towill LE. 1993. Pollen-handling protocol and hydration/dehydration characteristics of pollen for application to long-term storage. Euphytica 68:77–84.
Crowe JH, Crowe LM, Hoekstra FA, Wistrom CA. 1989. Effects of water on the stability of phospholipid biolayers: the problem of imbibition damage in dry organisms. Seed Moisture. CSSA Special Publication no 14:1–14.
Dafni A, Firmage D. 2000. Pollen viability and longevity: practical, ecological and evolutionary implications. Plant Systematics and Evolution 222:113–132.
Farmer RE, Barnett PE. 1974. Low-temperature storage of black walnut pollen. Cryobiology 11:366–367.
Firmage DH, Dafni A. 2001. Field tests for pollen viability: a comparative approach. Acta Horticulturae 561:87–94.
Ganeshan S. 1985. Cryogenic preservation of grape (Vitis vinifera L.) pollen. Vitis 24:169–173.
Ganeshan S. 1986a. Cryogenic preservation of papaya pollen. Scientia Horticulturae 28:65–70.
Ganeshan S. 1986b. Viability and fertilizing capacity of onion pollen (Allium cepa L.) stored in liquid nitrogen (–196°C). Tropical Agriculture (Trinidad) 63:46–48.
Ganeshan S, Alexander MP. 1986. Effect of freeze-drying on pollen germination in vitro in papaya (Carica papaya L. cv. Washington) and tomato (Lycopersicon esculentum Mill. Cv. Arka Vikas). Gartenbauwissenschaft 51:17–20.
Ganeshan S, Alexander MP. 1987. Storage and longevity of papaya (Carica papaya L. „Washington‟) pollen II. Effect of freeze-drying and storage at –20°C on pollen fertility. Gartenbauwissenschaft 52:207–209.
Ganeshan S, Alexander MP. 1990. Fertilizing ability of cryopreserved grape (Vitis vinifera L.) pollen. Vitis 29:145–150.
Ganeshan S, Alexander MP. 1991. Cryogenic preservation of lemon (Citrus limon Burm.) pollen. Gartenbauwissenschaft 56:228–230.
Ganeshan S, Rajashekaran RK. 2000. Current status of pollen cryopreservation research: relevance to tropical horticulture. In: Engelmann F, Takagi H, editors. Cryopreservation of Tropical Plant Germplasm. IPGRI, Rome. pp. 360–365.
Ganeshan S, Rajasekharan PE, Shashikumar S, Decruze, W. 2008. Cryopreservation of pollen In: Reed BM, editor. Plant Cryopreservation: A Practical Guide. Springer, New York. pp. 443–464.
Hanna WW, Towill LE. 1995. Long-term pollen storage. Plant Breeding Reviews 13:179–207.
Haunold A, Stanwood PC. 1985. Long-term preservation of hop pollen in liquid nitrogen. Crop Science 25:194–196.
Hauser EJP, Morrison JH. 1964. The cytochemical reduction of nitro blue tetrazolium as an index of pollen viability. American Journal of Botany 51:748–752.
Hecker RJ, Stanwood PC, Soulis CA. 1986. Storage of sugarbeet pollen. Euphytica 35:777–783.
Heslop-Harrison JS. 1992. Cytological techniques to assess pollen quality. In: Cresti M, Tiezzi A, editors. Sexual Plant Reproduction. Springer Verlag, Heidelberg. pp. 41–48.
Heslop-Harrison J, Heslop-Harrison Y. 1970. Evaluation of pollen viability by enzymatically induced fluorescence: intracellular hydrolysis of fluorescein diacetate. Strain Technology. 45:115–120.
Heslop-Harrison J, Heslop-Harrison Y, Shivanna KR. 1984. The evaluation of pollen quality, and a further appraisal of the fluorochromatic (FCR) test procedure. Theoretical and Applied Genetics 67:367–375.
Hoekstra FA. 1986. Water content in relation to stress in pollen. In: Leopold AC, editor. Membranes, Metabolism and Dry Organisms. Cornell University Press, Ithaca, New York. pp. 102–122.
Hoekstra FA. 1992. Stress effects on the male gametophyte. In: Cresti M, Tiezzi A, editors. Sexual Plant Reproduction. Springer Verlag Berlin. pp. 193–201.
Hoekstra FA 1995. Collecting pollen for genetic resources conservation. In: Guarino L, Ramanatha Rao V, Reid R, editors. Collecting Plant Genetic Diversity: Technical Guidelines, CAB International, Wallingford, UK. pp. 527–550.
Hoekstra FA. 2005. Differential longevities in desiccated anhydrobiotic plant systems. Integrated and Comparative Biology 45:725–733.
Hoekstra FA, Van der Wal EG. 1988. Initial moisture content and temperature of imbibition determine extent of imbibitional injury in pollen. Journal of Plant Physiology 133:257–262.
Hoekstra FA, Crowe LM, Crowe JH. 1989. Differential desiccation sensitivity of corn and Pennisetum pollen linked to their sucrose contents. Plant Cell and Environment 12:83–91.
Hoekstra FA, Crowe JH, Crowe LM. 1992. Germination and ion leakage are linked with phase transitions of membrane lipids during imbibition of Typha latifolia L. pollen. Physiologia Plantarum 84:29–34.
Hughes HG, Lee CW, Towill LE. 1991. Low-temperature preservation of Clianthus formosus pollen. HortScience 26:1411–1412.
Karipidis C, Olympios C, Passam HC, Savvas D. 2007. Effect of moisture content of tomato pollen stored cryogenically on in vitro germination, fecundity and respiration during pollen tube growth. Journal of Horticultural Science & Biotechnology 82:29–31.
Khatun S, Flowers TJ. 1995. The estimation of pollen viability in rice. Journal of Experimental Botany 46:151–154.
Lockwood DR, Richards CM, Volk GM. 2006. Wild plant sampling strategies: the roles of ecology and evolution. Plant Breeding Reviews 29:285–313.
Luza JG, Polito VS. 1987. Effects of desiccation and controlled rehydration on germination in vitro of pollen of walnut (Juglans spp.). Plant, Cell and Environment 10:487–492.
Marquard RD. 1992. Fruit set of pecan requires a low percentage of live pollen in controlled pollination. HortScience 27:473.
Martínez-Gómez P, Gradiel TM, Ortega E, Dicenta F. 2002. Low temperature storage of almond pollen. HortScience 37:691–692.
Namkoong G. 1981. Methods of pollen sampling for gene conservation. In: Franklin EC, editor. Pollen Management Handbook. USDA Agriculture Handbook No. 587. USDA, Washington DC. pp.74–76.
Ng NQ, Daniel IO. 2000. Storage of pollens for long-term conservation of yam genetic resources. In: Engelmann F, Takagi H, editors. Cryopreservation of Tropical Plant Germplasm. IPGRI, Rome. pp. 136–139.
Norton JD. 1966. Testing of plum pollen viability with tetrazolium salts. Proceedings of the American Society for Horticultural Science 89:132–134.
Pammenter NW, Vertucci CW, Berjak P. 1991. Homeohydrous (recalcitrant) seeds: dehydration, the state of water and viability characteristics in Landolphia kirkii. Plant Physiology 96:1093–1098.
Panella L, Wheeler L, McClintock ME. 2009. Long-term survival of cryopreserved sugarbeet pollen. Journal of Sugar Beet Research 46:1–9.
Parfitt DE, Almehdi AA. 1983. Cryogenic storage of grape pollen. American Journal of Enology and Viticulture 34:227–228.
Parfitt DE, Almehdi AA. 1984a. Cryogenic storage of olive pollen. Fruit Varieties Journal 38:14–16.
Parfitt DE, Almehdi AA. 1984b. Liquid nitrogen storage of pollen from five cultivated Prunus species. HortScience 19:69–70.
Parfitt DE, Ganeshan S. 1989. Comparison of proceduresfor estimating viability of Prunus pollen. HortScience 24:354–356.
Parton E, Deroose R, De Proft MP. 1998. Cryostorage of Aechmea fasciata pollen. CryoLetters 19:355–360.
Parton E, Vervaeke I, Delen R, Vandenbussche B, Deroose R, De Proft M. 2002. Viability and storage of bromeliad pollen. Euphytica 125:155–161.
Perveen A, Khan SA. 2008. Maintenance of pollen germination capacity of Malus pumila L. (Rosaceae). Pakistan Journal of Botany 40:963–966.
Pline WA, Edmisten KL, Oliver T, Wilcut JW, Wells R, Allen NS. 2002. Use of digital image analysis, viability stains, and germination assays to estimate conventional and glyphosate-resistant cotton pollen viability. Crop Science 42:2193–2200.
Priestley DA. 1986. Seed aging: implications for storage and persistence in the soil. Comstock Associates, Ithaca, New York. pp. 1–304.
Rajasekharan PE, Ganeshan S. 1994. Freeze preservation of rose pollen in liquid nitrogen: feasibility, viability and fertility status after long-term storage. Journal of Horticultural Science 69:565–569.
Rajasekharan PE, Rao TM, Janakiram T, Ganeshan S. 1994. Freeze preservation of gladiolus pollen. Euphytica 80:105–109.
Rodriguez-Riano T, Dafni A. 2000. A new procedure to assess pollen viability. Sexual Plant Reproduction 12:241–244.
Sacks EJ, St. Clair DA. 1996. Cryogenic storage of tomato pollen: effect on fecundity. HortScience 31:447–448.
Sato S, Katoh N, Iwai S, Hagimori M. 1998. Establishment of reliable methods of in vitro pollen germination and pollen preservation of Brassica rapa (syn. B. campestris). Euphytica 103:29–33.
Schoenike RE, Bey CF. 1981. Conserving genes through pollen storage. In: Franklin EC, editor. Pollen Management Handbook. USDA Agriculture Handbook No. 587. USDA, Washington DC. pp.72–73.
Sedgley M. 1981. Storage of avocado pollen. Euphytica 30:595–599.
Shivanna KR. 2003. Pollen Biology and Biotechnology. Science Publishers, Enfield, New Hampshire. pp. 204–210.
Shivanna KR, Heslop-Harrison J. 1981. Membrane state and pollen viability. Annals of Botany 47:759–770.
Shivanna KR, Linskens HF, Cresti M. 1991. Pollen viability and pollen vigor. Theoretical and Applied Genetics 81:38–42.
Sparks D, Yates IE. 2002. Pecan pollen stored over a decade retains viability. HortScience 37:176–177.
Tandon R, Chaudhury R, Shivanna KR. 2007. Cryopreservation of oil palm pollen. Current Science 92:182–183.
Tisserat B, Ulrich JM, Finkle BJ. 1983. Survival of phoenix pollen grains under cryogenic conditions. Crop Science 23:254–256.
Towill LE. 1981. Liquid nitrogen preservation of pollen from tuber-bearing Solanum species. HortScience 16:177–179.
Towill LE. 1985. Low temperature and freeze-/vacuum-drying preservation of pollen. In: Kartha KK, editor. Cryopreservation of Plant Cells and Organs. CRC Press, Boca Raton, Florida. pp. 171–198.
Towill LE. 2004. Pollen storage as a conservation tool. In: Guerrant EO, Havens K, Maunder M, editors. Ex Situ Plant Conservation: Supporting Species Survival in the Wild. Island Press, Washington DC. pp.180–188.
Tyagi RK, Hymowitz T. 2003. Pollen from Glycine species survive cryogenic exposure. CryoLetters 24:119–124.
Van Bilsen DGJL, Hoekstra FA, Crowe LM, Crowe JH. 1994. Altered phase behaviour in membranes of aging dry pollen may cause imbibitional leakage. Plant Physiology 104:1193–1199.
Van der Walt ID, Littlejohn GM. 1996. Storage and viability testing of Protea pollen. Journal of the American Society for Horticultural Science 121:804–809.
Yates IE, Sparks D. 1990. Three-year-old pecan pollen retains fertility. Journal of the American Society for Horticultural Science 115:359–363.
Yates IE, Sparks D, Connor K, Towill L. 1991. Reducing pollen moisture simplifies long-term storage of pecan pollen. Journal of the American Society for Horticultural Science 116:430–434.
Zhang LX, Chang WC, Wei YJ, Liu L, Wang YP. 1993. Cryopreservation of ginseng pollen. HortScience 28:742–743.
Chapter 20: Collecting and handling seeds in the field
F. R. Hay
International Rice Research Institute, Metro Manila, Philippines
E-mail: f.hay(at)cgiar.org
R. J. Probert
Royal Botanic Gardens, Kew, Ardingly, Nr. Haywards Heath, West Sussex, UK
E-mail: r.probert(at)kew.org
|
2011 version |
1995 version |
||
|
Open the full chapter in PDF format by clicking on the icon above. |
|||
This chapter is a synthesis of new knowledge, procedures, best practices and references for collecting plant diversity since the publication of the 1995 volume Collecting Plant Genetic Diversity: Technical Guidelines, edited by Luigi Guarino, V. Ramanatha Rao and Robert Reid, and published by CAB International on behalf of the International Plant Genetic Resources Institute (IPGRI) (now Bioversity International), the Food and Agriculture Organization of the United Nations (FAO), the World Conservation Union (IUCN) and the United Nations Environment Programme (UNEP). The original text for Chapter 20: Collecting and Handling Seeds in the Field, authored by R. D. Smith, has been made available online courtesy of CABI. The 2011 update of the Technical Guidelines, edited by L. Guarino, V. Ramanatha Rao and E. Goldberg, has been made available courtesy of Bioversity International.
Please send any comments on this chapter using the Comments feature at the bottom of this page. If you wish to contribute new content or references on the subject please do so here.
Back to list of chapters on collecting
Internet resources for this chapter
Abstract
 |
|
|
Appropriate handling of immature fruits can greatly improve seed quality for long-term storage. |
Seed banking remains a cornerstone in the conservation of plant genetic resources. To be successful, it relies on the collecting and banking of high-quality seeds. In 1995 there was relatively little guidance available to seed collectors (especially of wild plant species), who had to make decisions in the field about what to collect and how to handle collected material. Since then, research into various aspects of seed conservation has meant collectors should have a better idea of the storage behaviour and potential longevity of seeds of a target species and the likely maturity status of the collected seeds. Knowledge of the moisture status of the seeds, which can be quickly and easily determined in situ, and of the local climatic conditions, can help inform what to do with a collection immediately after harvest. A particular concern is that, while a relatively small proportion of species were known to have recalcitrant or intermediate seed storage behaviour, there may be many technically orthodox species whose seeds are so short-lived in conventional seed bank storage that alternative storage conditions or even methods of conservation will have to be sought.
Introduction
In the earlier version of this chapter, published in 1995, Roger Smith concluded that there was a scarcity of published data to aid collectors on the post-harvest handling of seeds. Shortly after that, Roger was responsible for leading the Millennium Seed Bank Project, the largest seed conservation effort for wild plant species ever undertaken. It is therefore no coincidence that a major thrust of research on seed storage behaviour, longevity, development and post-harvest handling began around that time.
In 1995, we knew that the moisture status of seeds at the time of collection and during the post-harvest period would have a major impact on subsequent seed longevity. Historical climate data could be used to predict the likely affects on seed longevity if seeds were exposed to average ambient conditions in the field. However, portable and reliable means of directly measuring seed moisture status and ambient conditions in the field were not widely available at that time; they are now and are already used by seed collectors around the globe.
Our knowledge of the relationship between seed maturity and the development of seed tolerance to desiccation and seed longevity is now also greatly improved, which has enabled the development of clear practical guidelines for post-harvest handling. We also have a much better understanding of the variation among species in inherent seed longevity and the factors that correlate with such differences. This good news, however, is tempered by the fact that we have now discovered wild plant species and crop relatives that are potentially extremely short lived using conventional techniques for seed conservation.
The global threat to plant diversity from land conversion, invasive species and climate change means that it is now more important than ever to collect and conserve plant genetic resources. Armed with the knowledge gained and tools developed since 1995, collectors can be more confident that collections will reach the seed bank in prime condition.
Current Status
Seed storage types
One of the first considerations when deciding how to collect and handle seeds of a particular species is storage behaviour. The Seed Information Database (SID) (http://data.kew.org/sid) began as an online version of the Compendium of Information on Seed Storage Behaviour (Hong et al. 1998), which categorized species as having recalcitrant, intermediate or orthodox seed storage behaviour based on published data and unpublished seed storage results from the Seed Bank of the Royal Botanic Gardens Kew. SID currently contains information on the seed storage behaviour of 19,676 species and can be searched at different levels of taxon (although not by common names). Most species for which information is available (93.9%) are described as having orthodox (or probably/likely orthodox) seed storage behaviour, although as observed previously, the species included in SID are probably biased towards temperate and/or useful plant species that might be more likely to have orthodox seed storage behaviour. Only 2.8% are described as having recalcitrant (or probably/likely recalcitrant) seed storage behaviour. The remaining species (0.8%) fall into the intermediate category of seed storage behaviour or have uncertain storage behaviour (based on the available data).
Where information is not found in SID or through other literature or web searching, it may be necessary to empirically determine seed storage behaviour, if seeds, time and facilities are available. Hong and Ellis (1996) presented an experimental scheme to determine seed storage behaviour whereby seeds are dried to increasingly lower moisture contents and tested for germination; samples of those that survive drying to 10% moisture content are further dried and stored at -18°C for three months and then tested again. This scheme requires a large number of seeds. Pritchard et al. (2004) described a simpler version where small samples of seeds are used for moisture content and germination testing before and after drying; drying to equilibrium with silica gel is monitored by following the change in seed weight. These types of experiments on desiccation tolerance assume that appropriate dormancy-breaking treatments and germination requirements are known. This might not be the case, and for example, as reported for Carica papaya, dormancy can be induced by drying (Wood et al. 2000). Similarly, if the low temperature response as well as the desiccation response is tested, as in the Hong and Ellis (1996) schematic, it should be noted that the low temperature might reduce germination for reasons other than loss of viability, per se. Crane et al. (2003) found that there was reduced germination of seeds of some Cuphea species due to the crystallization of particular fatty acids within the seeds. Higher germination was achieved if the seeds were given a heat pulse to melt these lipids before imbibition (a response that cannot be described as the induction and release of dormancy).
Recalcitrant seed storage behaviour is still expected to be more prevalent in the warm moist habitats of tropical and sub-tropical forests, compared with more arid areas, and these seeds tend to have particular characteristics: they are likely to be non-dormant, dispersed at a high seed moisture content (since they are metabolically active) and during the wettest months of the year, relatively large and likely to have relatively thin outer tissues (endocarp and testa) (Daws et al. 2005; Ellis et al. 2007; Berjak and Pammenter 2008). Daws et al. (2006) used these last two traits in a model that can be used to predict the likelihood of desiccation sensitivity. If there is a high probability of desiccation sensitivity, it would be unwise to make a large seed collection for conventional seed bank storage. The expected ‘hot spot’ of desiccation intolerance among aquatic species has not been entirely borne out. Hay et al. (2000) found that a considerable number (65) of aquatic plants native to the UK do produce seeds that could withstand desiccation; only nine species were described as having recalcitrant or intermediate storage behaviour based on levels of desiccation tolerance. Similarly, although less surprising, Tuckett et al. (2010) reported that seeds of aquatic species found in temporary pools of Western Australia have orthodox storage behaviour.
The extent of desiccation tolerated by seeds of species that are classified as having recalcitrant storage behaviour varies. This has led to the theory of a continuum of seed storage behaviour (for a review see Berjak and Pammenter 2008), from highly recalcitrant species, whose seeds tolerate little or no desiccation, through to extremely orthodox species, whose seeds are highly desiccation tolerant and long lived. Desiccation tolerance can even vary between different seed lots within a species and between individual seeds within a seed lot. Daws et al. (2004) showed how the degree of desiccation tolerance varied between seed lots of the recalcitrant species Aesculus hippocastanum, depending on provenance within its European range; differences were attributed to enhanced development of the seeds in warmer environments. Similarly for tropical species, the level of ‘maturity’ of the seed at shedding may determine the level of desiccation tolerated (Berjak et al. 1993; Lin and Chen 1995). Between species it has been observed that the slower the rate of water loss, the greater the desiccation sensitivity (Berjak and Pammenter 2008). Berjak and Pammenter (2008) also gave practical advice for the conservation of recalcitrant seeds: seeds should be kept at their harvest (or shedding) moisture content and at the lowest temperature that does not incur chilling damage. Seeds of species from temperate regions may tolerate lower temperatures (0-5°C) than species from tropical regions. Provided that fungal growth can be controlled, recalcitrant seeds may remain viable under such conditions for several months to a year or two, at best. For long-term ex situ conservation, cryopreservation techniques, often of excised embryonic axes, remains the only option for desiccation-sensitive species, including those that have been termed ‘intermediate’ but which might also be considered, on the continuum of desiccation tolerance, as ‘minimally recalcitrant’.
Desiccation-tolerant (orthodox) seeds
Orthodox seed development
The timing of seed collection is important since seed quality increases late in seed development: during the desiccation stage after the attainment of mass maturity (maximum seed dry weight). (Note that for orthodox seeds borne in fleshy fruits, there may be limited loss of water from the developing seed.) Understanding the physiology and patterns of gene expression of this stage of seed development continues to be a focus of seed research (for a review see Angelovici et al. 2010).
Seeds of many orthodox species acquire desiccation tolerance around the time of mass maturity, some time before the desiccation stage is completed and seeds, in the case of wild species, are dispersed. However, there are some seemingly orthodox species in which there is little or no desiccation phase before seed dispersal, and some individual seeds within a cohort that might be considered ready for collection (on the point of natural dispersal) might not be fully desiccation tolerant. This phenomenon has been observed in the spring-flowering herbaceous geophyte, Anemone nemorosa, where only 30% of the freshly harvested seeds survived drying for 21 days at 15% relative humidity (RH), 15°C (Ali et al. 2007). Desiccation tolerance increased during the first five days after harvest in seeds placed on agar at 20°C. This Anemone nemorosa data shows how seed maturity at harvest can vary between individual seeds. Hay et al. (2010) attempted to determine the sources of variability in seed maturity by following the development of a cohort of seeds of Trifolium ambiguum. They concluded that, for this species, seed-to-seed variability in the timing of the onset of germinability, desiccation tolerance and hardseededness, and in the gaining of seed longevity was inevitable. Seed collectors in the field, even when collecting from a crop species where there might be less variability in phenology and micro-environment between individual plants, are unlikely to be able to make a collection of seeds with completely uniform maturity. Attainment of maximum seed longevity in T. ambiguum was associated with a change in seed coat colour from orange to dark orange, although there were subsequent declines in potential longevity (Hay et al. 2010). A useful review of field markers of seed maturity, including changes in fruit and seed coat colour can be found in Hay and Smith (2003). Another potential marker of seed maturity is the amount of chlorophyll fluorescence emitted by the intact seeds since, for some species, the amount of chlorophyll in the seeds declines during seed development (Jalink et al. 1998). Whether such a technology could actually be of practical use on a seed-collecting expedition remains to be seen, but seed sorters that discriminate on the basis of either colour or chlorophyll fluorescence are available and could be used during the processing of seed accessions (Dell’Aquilla 2009).
Moisture content is also commonly used to assess seed maturity. However, empirical determination of seed moisture content is a destructive test and obviously requires laboratory facilities. Probert (2003) described how portable instruments that determine the equilibrium relative humidity (eRH)—the RH of the air around a sample when the system is in equilibrium—of a seed sample can be used to decide whether it is appropriate to make a collection or, if a collection is made, how the seeds should be processed. The use of digital hygrometers and alternatives for measuring seed moisture status has been described in more detail by Probert et al. (2003). These methods and practical guidelines for post-harvest handling of seeds (see below) are also covered by Millennium Seed Bank information sheets 04, 05, and 07 (www.kew.org/msbp/scitech/publications/info_sheets.htm). In dry climates, if the eRH of a sample of seeds is already close to ambient RH, seeds should be collected as soon as possible. If fruits/seeds do not easily detach from the maternal plant and the eRH of the seeds is still high (85%–100%), it is better to wait for further maturation before making a collection.
Handling seeds in the field
How seeds are handled immediately after harvest is critical to their subsequent longevity in storage. Particular care should be taken when a species’ seeds are expected to be extremely short-lived (see below). The timing of seed collection is also important; however, it might not always be possible to wait to collect seeds on the point of natural dispersal (as recommended for seeds of wild species) because of logistical constraints.
If fruits/seeds have a very high eRH at harvest (85%–100%) and if resources are available to create a controlled, non-desiccating environment, they should not be rapidly dried. Seed quality is likely to increase if such seeds are held under conditions (temperature, humidity) that are close to the conditions that they would experience if they remained on the maternal plant (Probert et al. 2007). As a general rule, suitable conditions to aim for would be ~75% RH and close to ambient temperature. During a collecting expedition, it might be more practical to simply hold intact fruits under shaded ambient conditions to allow continued ripening (table 20.1). Butler et al. (2009) found that if seeds are collected before the end of the desiccation phase of seed development and dried, developmental processes leading to increases in seed quality might be resumed if seeds are later placed at high humidity. This may be worth considering in situations where it has not been possible to hold seeds at high humidity immediately after harvest, although it needs validating for more species before it might be considered appropriate for routine use.
Table 20.1. Decision-Making Framework for Post-Harvest Handling of Orthodox Seeds from Non-Fleshy Fruits during Seed Collecting Missions, Based on an Understanding of Ambient Conditions and Seed Moisture Status
|
Seed Maturity Stage |
Seed Moisture Status |
Ambient Conditions |
|
|
Dry |
Humid |
||
|
Immature |
Wet (85% to 100% eRH) |
Hold intact fruits under shaded ambient conditions for 1-2 weeks for continued ripening. |
|
|
At natural dispersal |
Damp > 50% eRH |
Dry in a thin layer, in a well-ventilated location. Minimise moisture absorption at night. |
Transfer to seed bank as soon as possible or dry with a desiccant such as silica gel or place in an air-conditioned room. |
|
Dry < 50% eRH |
Hold in loosely packed mesh or paper bags in a well-ventilated, shaded location. Minimize moisture absorption at night. |
||
Note: For more details, see MSBP technical information sheets 04 and 05 (www.kew.org/msbp/scitech/publications/info_sheets.htm).
If the eRH of collected seeds is between 50% and 85%, the rate of aging is likely to be unacceptable. If the ambient conditions are also hot and humid, it will be necessary to dry the seeds as soon as possible using a desiccant and/or transfer the seeds to an air-conditioned or purpose-built dry room. In less humid climates, the seeds can be dried by placing them in a thin layer in a well-ventilated, shaded location. Seeds should not be allowed to take up moisture overnight when ambient air humidity increases. This can be prevented by sealing them in air-tight containers. For seeds that are already relatively dry at the time of collection, the advice is similar except that in dry climates, the seeds can be loosely packed in mesh or paper bags and kept in a ventilated location (table 20.1).
The eRH of fleshy fruits is likely to be high regardless of the maturity stage of the seeds inside, and there is little point in measuring the eRH of these fruits. Seeds from such fruits should be extracted as soon as physical signs (such as fruit colour) suggest that the fruits are ripe. Unripe fruits should be kept under ambient conditions and direct sunlight may not be unfavourable. Simulating natural conditions, extracted seeds should also be allowed to dry relatively slowly (i.e., aiming for the ~75% RH as above) before being transferred to the genebank dry room.
Variation in the longevity of orthodox seeds
Even if a species falls into the orthodox category of seed storage behaviour in that the seeds are desiccation tolerant, it does not mean that conventional seed bank storage is an appropriate method of ex situ conservation. There is increasing evidence that the seeds of some wild species are extremely short-lived in air-dry storage. For example, seeds of Anemone nemorosa are predicted to survive only a year or two at most under conventional seed bank conditions (Ali et al. 2007). This finding is consistent with those of Probert et al. (2009), who found that relatively short seed longevity in air-dry storage was correlated with the presence of endosperm and with cool, wet environments. Walters et al. (2005) similarly found taxonomic and climatic trends in relation to seed longevity among accessions (breeding lines, landraces, and wild populations) comprising 276 species, including many common crops, and Mondoni et al. (2011) have recently shown that seeds of Alpine plants were significantly shorter lived than related species and ecotypes from nearby lowland habitats. If collected seeds are expected to be very short-lived in air-dry storage, care is needed to ensure that viability is not lost before seeds arrive at the seed bank (see table 20.1). Li and Pritchard (2009) have suggested that it makes economic sense to use ultra-cold storage (in or above liquid nitrogen) for short-lived (indeed all) orthodox seeds, particularly for threatened and vulnerable wild species. At the extreme end of the orthodox scale, Probert et al. (2009) found that seeds from some Australian species were particularly long-lived, and Sallon et al. (2008) have reported germination of date seeds (Phoenix dactylifera L.) that were carbon dated to be about 2000 years old. Delays in the processing of such seeds would be less detrimental than for short-lived seeds, although best practice should nonetheless be followed.
The Ellis and Roberts (1980) improved viability equation remains a useful tool for predicting seed longevity in air-dry storage and also the potential losses that might be incurred if processing (drying and storage) is delayed, if the ‘viability constants’ have been determined for the species of interest. SID has a useful module that can be used to predict viability loss for species for which the viability constants of the equation have been determined. Currently, constants for 56 species are available in the database, including common cereal and legume crops as well as wild species. Constants for other species may be available in the literature, and the values can be entered within the seed-viability constants module of SID to make predictions. The module also allows the user to estimate equilibrium moisture content if the seed oil content for the species is known. Determining species viability constants for other species is relatively simple if the Dickie et al. (1990) universal values are used for the temperature constants, requiring experimental storage of seed samples to be carried out using a range of moisture contents at a single temperature (cf. a range of moisture contents and temperatures).
Future challenges/needs/gaps
The process of seed collecting always sounds as if it should be quite straightforward. However, in truth, if the seeds are to be of value as a genetic resource, we know that considerable care is needed in terms of deciding when and whether to collect and how to handle the seeds in the field. Collectors would still benefit from the development of fast, reliable tools for use in the field to diagnose seed storage behaviour, maturity stage, and perhaps, for orthodox seeds, potential longevity. Portable systems for efficient drying of seeds during a collecting trip or, if appropriate the converse, for holding them at high humidity, also need to be improved. More research is needed on potentially difficult and understudied groups: for example, temperate woodland geophytes, aquatic species and alpine species, whose seeds might be desiccation tolerant but very short-lived in conventional seed bank storage. Such studies might consider optimum time to collect, appropriate drying environments, treatments to overcome germination problems and dormancy, and alternative storage conditions.
Non-orthodox seeds still present considerable challenges, not least since a wide variety of species, including some important crops and/or their wild relatives, have seeds that are non-orthodox.
Conclusions
Key points restated and updated.
For all seeds
-
Attempt to collect equal numbers of seeds from each plant sampled at the time of natural seed dispersal. Do not collect from the ground unless you can be sure seeds have only recently dispersed. Avoid damaged seeds (mechanical damage, pest attack).
-
If seeds must be cleaned during the trip, do so by hand to minimize the chance of mechanical damage.
-
Plan your activities so that no more than one month elapses between collecting and reception by the seed bank.
-
If it is possible to avoid quarantine seed treatments without breaking quarantine regulations (for example through post-entry quarantine), do so.
-
Personally ensure that seed arrives at the seed bank without undue delay. International air-freight companies offer a reliable service for sending seed batches around the world. Major companies provide online tracking services, and packages travel in pressurized, temperature-controlled aircraft cabins so there is little risk to seed quality during transit.
For desiccation-intolerant seeds
-
Keep seeds aerated and moist in inflated polythene bags, changing the air at least weekly by deflation and re-inflation.
-
Do not allow such seeds collected in the tropics to either cool below 20°C or heat up above ambient shade temperatures in the field or during transport.
For desiccation-tolerant seeds or their fruits
-
Make direct measurements of seed moisture status and ambient conditions at the time of collection using a suitable hygrometer to assess the risk of significant loss in seed viability during transit. Use the results to inform post-harvest handling decisions (table 20.1).
-
For fleshy-fruited species, if logistically possible consider field cleaning of fully ripe fruits followed by shade (slow) drying of extracted seeds for three days or more (larger seeds need longer) to reduce the seed moisture content towards equilibrium with ambient relative humidity before packing. If the fruits are not fully ripe, then keep the fruits intact and aerated, at ambient temperatures.
-
For fruits that are dry dehiscent or indehiscent, it is usually preferable to keep the fruits intact and aerated, at ambient temperatures. However, to use space more efficiently, seeds could be extracted by hand and dried in the shade as above.
-
Collections containing a significant proportion of immature seeds or fruits should be allowed to continue ripening under ambient conditions but protected from exposure to direct sunlight. Such collections should be regularly checked for physical indications of improved maturity.
-
If the collection comprises morphologically distinct maturity stages, consideration should be given to splitting the collection and treating the immature and mature seeds differently.
Back to list of chapters on collecting
References and further reading
Ali N, Probert R, Hay F, Davies H, Stuppy W. 2007. Post-dispersal embryo growth and acquisition of desiccation tolerance in Anemone nemorosa L. seeds. Seed Science Research 17:155–163.
Angelovici R, Galili G, Fernie AR, Fait A. 2010. Seed desiccation: a bridge between maturation and germination. Trends in Plant Science 15:211–218.
Berjak P, Pammenter NW. 2008. From Avicennia to Zizania: seed recalcitrance in perspective. Annals of Botany 101:213–228.
Berjak P, Vertucci CW, Pammenter NW. 1993. Effects of developmental status and dehydration rate on characteristics of water and desiccation sensitivity in recalcitrant seeds of Camellia sinensis. Seed Science Research 3: 155–166.
Butler LH, Hay FR, Ellis RH, Smith RD. 2009. Post-abscission pre-dispersal seeds of Digitalis purpurea L. remain in a developmental state that is not terminated by desiccation ex planta. Annals of Botany 103:785–794.
Crane J, Miller AL, van Roekel JW, Walters C. 2003. Triacylglycerols determine the unusual storage physiology of Cuphea seed. Planta 217:699–708.
Daws MI, Garwood NC, Pritchard HW. 2005. Traits of recalcitrant seeds in a semi-deciduous tropical forest in Panamá: some ecological implications. Functional Ecology 19:874–885.
Daws MI, Garwood NC, Pritchard HW. 2006. Prediction of desiccation sensitivity in seeds of woody species: a probabilistic model based on two seed traits and 104 species. Annals of Botany 97:667–674.
Daws MI, Lydall E, Chmielarz P, Leprince O, Matthews S, Thanos CA, Pritchard JW. 2004. Developmental heat sum influences recalcitrant seed traits in Aesculus hippocastanum across Europe. New Phytologist 162:157–166.
Dell’Aquila A. 2009. Development of novel techniques in conditioning, testing and sorting seed physiological quality. Seed Science & Technology 37:608–624.
Dickie JB, Ellis RH, Kraak HL, Ryder K, Tompsett PB. 1990. Temperature and seed storage longevity. Annals of Botany 65:197–204.
Ellis RH, Roberts EH. 1980. Improved equations for the prediction of seed longevity. Annals of Botany 45:13–30.
Ellis RH, Mai-Hong T, Hong TD, Tan TT, Xuan-Chuong ND, Hung LQ, Ngoc-Tam B, Le-Tam VT. 2007. Comparative analysis by protocol and key of seed storage behaviour of sixty Vietnamese tree species. Seed Science & Technology 35:460–476.
Hay FR, Smith RD. 2003. Seed maturity: when to collect seeds from wild plants. In: Smith RD, Dickie JB, Linington SH, Pritchard HW, Probert RJ, editors. Seed Conservation: Turning Science into Practice. Royal Botanic Gardens, Kew, UK. pp. 97–133.
Hay F, Probert R, Marro J, Dawson M. 2000. Towards the ex situ conservation of aquatic angiosperms: a review of seed storage behaviour. In: Black M, Bradford KJ, Vázquez-Ramos J, editors. Seed Biology: Advances and Applications. CAB International, Wallingford, UK. pp. 161–177.
Hay FR, Smith RD, Ellis RH, Butler LH. 2010. Developmental changes in the germinability, desiccation tolerance, hardseededness, and longevity of individual seeds of Trifolium ambiguum. Annals of Botany 105:1035–1052.
Hong TD, Ellis RH. 1996. A Protocol to Determine Seed Storage Behaviour. IPGRI Technical Bulletin No. 1. IPGRI, Rome.
Hong TD, Linington SH, Ellis RH. 1998. Compendium of Information on Seed Storage Behaviour, Volumes I and II. Royal Botanic Gardens, Kew, UK.
Jalink H, van der Schoor R, Frandas A, van Pijlen, Bino RJ. 1998. Chlorophyll fluorescence of Brassica oleracea seeds as a non-destructive marker for seed maturity and seed performance. Seed Science Research 8:437–443.
Li D-Z, Pritchard HW. 2009. The science and economics of ex situ plant conservation. Trends in Plant Science 14:614–621.
Lin TP, Chen M-H. 1995. Biochemical characteristics associated with the development of the desiccation-sensitive seeds of Machilus thunbergii Sieb. & Zucc. Annals of Botany 76:381–387.
Mondoni A, Probert RJ, Rossi G, Vegini E, Hay FR. 2011. Seeds of alpine plants are short lived: implications for long-term conservation. Annals of Botany 107:171–179.
Pritchard HW, Wood CB, Hodges S, Vautier HJ. 2004. 100-seed test for desiccation tolerance and germination: a case study on eight tropical palm species. Seed Science & Technology 32:393–403.
Probert RJ. 2003. Seed viability under ambient conditions, and the importance of drying. In: Smith RD, Dickie JB, Linington SH, Pritchard HW, Probert RJ, editors. Seed Conservation: Turning Science into Practice. Royal Botanic Gardens, Kew, UK. pp. 337–365.
Probert RJ, Daws MI, Hay FR. 2009. Ecological correlates of ex situ seed longevity: a comparative study on 195 species. Annals of Botany 104:57–69.
Probert RJ, Manger KR, Adams J. 2003. Non-destructive measurement of seed moisture, In: Smith RD, Dickie JB, Linington SH, Pritchard HW, Probert RJ, editors. Seed Conservation: Turning Science into Practice. Royal Botanic Gardens, Kew, UK. pp. 367–387.
Probert R, Adams J, Coneybeer J, Crawford A, Hay F. 2007. Seed quality for conservation is critically affected by pre-storage factors. Australian Journal of Botany 55:326–355.
Sallon S, Solowey E, Cohen Y, Korchinsky R, Egli M, Woodhatch I, Simchoni O, Kislev M. 2008. Germination, genetics, and growth of an ancient date seed. Science 320:1464.
Tuckett RE, Merritt DJ, Hay FR, Hopper SD, Dixon KW. 2010. Comparative longevity and low-temperature storage of seeds of Hydatellaceae and temporary pool species of south-west Australia. Australian Journal of Botany 58:327–334.
Walters C, Wheeler LM, Grotenhuis JM. 2005. Longevity of seeds stored in a genebank: species characteristics. Seed Science Research 15:1–20.
Wood CB, Pritchard HW, Amritiphale D. 2000. Desiccation-induced dormancy in papaya (Carica papaya L.) seeds is alleviated by heat shock. Seed Science Research 10:135–145.
Seed Information Database (SID): http://data.kew.org/sid/
Millennium Seed Bank information sheets 04, 05 and 07: www.kew.org/msbp/scitech/publications/info_sheets.htm
Chapter 13: Published information resources for plant germplasm collectors
M. Garruccio
Bioversity International, Rome, Italy
E-mail: m.garruccio(at)cgiar.org
|
2011 version |
1995 version |
||
|
Open the full chapter in PDF format by clicking on the icon above. |
|||
This chapter is a synthesis of new knowledge, procedures, best practices and references for collecting plant diversity since the publication of the 1995 volume Collecting Plant Genetic Diversity: Technical Guidelines, edited by Luigi Guarino, V. Ramanatha Rao and Robert Reid, and published by CAB International on behalf of the International Plant Genetic Resources Institute (IPGRI) (now Bioversity International), the Food and Agriculture Organization of the United Nations (FAO), the World Conservation Union (IUCN) and the United Nations Environment Programme (UNEP). The original text for Chapter 13: Bibliographic Databases for Plant Germplasm Collectors, authored by J .A. Dearing and L. Guarino, has been made available online courtesy of CABI. The 2011 update of the Technical Guidelines, edited by L. Guarino, V. Ramanatha Rao and E. Goldberg, has been made available courtesy of Bioversity International.
Please send any comments on this chapter using the Comments feature at the bottom of this page. If you wish to contribute new content or references on the subject please do so here.
Back to list of chapters on collecting
Abstract
Widespread access to the internet and the technological changes we have seen over the last 16 years, since the first edition of this collecting manual, have dramatically altered the way people access and use information. They have also changed the paradigm of scientific publishing and research. Tools and resources for accessing information on plant genetic resources have grown and evolved from being mainly traditional and paper-based to sophisticated web-based platforms that provide the researcher with many features. Other tools, such as the social media platforms that have recently emerged, allow researchers to connect and collaborate with their peers regardless where they are located in the world. This paper looks at selective trends and movements that have had an impact on the paradigm of scientific publishing and research in the last 16 years, and, within this context, highlight authoritative web-based information resources for those working in the area of plant genetic resources.
|
Most websites nowadays offer direct access to the latest web tools and social media. |
Introduction
Sixteen years has passed since the publication of the first edition of Collecting Plant Genetic Diversity : Technical Guidelines. In that time, widespread access to the internet, along with other technological changes, have dramatically altered the way people access and use information. They have also changed the paradigm of scientific publishing and research. Sixteen years ago, a researcher would have primarily consulted hardcopies of abstract journals or standalone databases that were available at a library for his/her research purposes. The completed research would then be disseminated mainly via the medium of publishing in a scientific journal (available only in hardcopy). The same researcher would then meet and collaborate with peers, face to face, at conferences.
This scenario of only 16 years ago is difficult to grasp, when today, searching for information on search engines such as Google has become second nature, as has our ability to access, disseminate and generate information. The internet, today, can allow that same researcher to make his/her work available to the world, anywhere, at any time, in many different formats. It can also allow the researcher the possibility of collaborating with peers on different electronic platforms without leaving his/her desk.
While accessing the internet has now become part of our daily lives, the unprecedented amounts of information a researcher is faced with can be daunting. Together, published and professional web content is estimated to generate about 5 gigabytes/day, whereas user-generated content is created at the rate of about 10 gigabytes/day and growing. (Agichtein et al. 2009). These figures make it eminently clear that filters are required in order to access high-quality, authoritative scientific information. There is also a need to explore the concept that scientific research is now being carried out, communicated and disseminated by social media tools that previously did not exist. The researcher today has the opportunity to access information from many different formats, not just the traditional bibliographic databases that were discussed in the 1995 edition of these technical guidelines.
The two main objectives of this chapter are (1) to look at selective trends and movements that have had an impact on the paradigm of scientific publishing and research in the last 16 years and (2) within this context, to highlight authoritative web-based information resources for those working in the area of plant genetic resources.
Current status
The focus of this chapter is on the main trends and phenomena that have emerged over the last 16 years: (1) using search engines more effectively, (2) open-access resources, (3) social-media tools, (4) bibliographic databases and (5) reference-management systems.
Web searching: how to improve search results
Nowadays most people use the internet for their research purposes, but how effectively? If we are to examine the daunting statistics given to us by Agichtein et al. (2009), we realise that learning how search engines respond to queries can make a significant difference in the results we encounter when searching the web. Learning a few quick shortcuts and tricks can assist the researcher in finding more relevant and targeted information. Most of the major search engines have tutorials and guides on how to improve search results and retrieve relevant information. Table 13.1 provides a good overview of the search engines that are most used and where to locate their on-line tutorials and cheat sheets.
Table 13.1: Web Searching: Improving Your Search Results
This table provides information on where to locate search tips, shortcuts and cheat sheets for the three major search engines currently in use. Learning to use these tips will improve the relevance of research results.
|
Search engine |
Search tips, shortcuts and cheat sheets |
|
|
Using Yahoo! Search (covers simple, advanced searching, plus tips and preferences) |
||
|
Search tips and techniques |
||
Note: Access date for this information was 30 June 2011.
Google is the most used search engine. As of December 2010, its market share was 90.57% (StatCounter 2011). Nancy Blachmann from GoogleGuide.com has developed a simple and clear overview of the responses Google will provide depending on how one formulates the query (figure 13.1).
These searching tips can also be used when searching across portals such as Google Scholar, which is one of the major free web search engines that indexes the full text of mainly scholarly literature across an array of disciplines. It allows the researcher to search across many sources (e.g., professional societies, universities, academic publishers) from just one place, and one can view papers and documents either in full text or with a limited preview, depending on the content provider and how much they want their content to be freely available. Google Scholar is one of the first places many researchers refer to when embarking on specific fact-finding.
 |
|
Figure 13.1: Formulating queries for Google |
In some cases, it might also be relevant to search specifically for news items on different PGR-related issues, rather than websites in general. Strategies for doing this are discussed on the Google Books portal.
“Open” information resources: scholarly literature
The movement in scholarly publishing, known as open access, became much more prominent in the 1990s with the advent of the internet. It is a topic that in recent years has become the subject of much discussion among the academic community, funding agencies, government officials and publishers. "By 'open access' …we mean its free availability on the public internet, permitting any users to read, download, copy, distribute, print, search, or link to the full texts of these articles, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose, without financial, legal, or technical barriers other than those inseparable from gaining access to the internet itself” (Suber 2011).
Open-access scientific content is accessed in two primary ways: through open-access repositories (digital collections) where researchers archive or deposit their post-print, or via open-access journals and e-books. While open access was a term hardly heard of 16 years ago, a study carried out by Björk et al. (2010) revealed that 20% of peer-reviewed articles across all disciplines are now freely available over the web, and this figure will continue to grow as authors and their affiliated organizations begin to understand the benefits that open access can bring.
One such benefit is higher citations: open-access papers are more cited than papers that are behind a “payment wall” ( Hajjem and Harnad 2005; Harnad and Brody 2004; Swan 2010). Higher citations important for many universities or research organizations that want to improve their research impact and prestige. Yet, at the same time, content that is open access is often misunderstood, and at times the quality of the science published as open access is seen as somewhat inferior; however, as Swan (n.d.) succinctly states, “Open access is not self-publishing, nor a way to bypass peer-review and formal publication, nor is it a kind of second-class, cut-price publishing route. It is simply the means to make research results freely available online to the whole research community.” In fact, many open-access scientific journals are widely read and have impressive impact factors; the journal PLOS Biology has an impact factor of 12.469 (Thomson Reuters 2011)
There are numerous “open” information resources and platforms that are available on the internet for researchers working in plant genetic resources, where it is possible to access full-text research papers. Content is immediate, online and freely available without the use restrictions commonly imposed by publishers. Another major benefit open access brings is that it has removed the cost barrier for accessing scientific information, particularly for researchers and libraries in developing countries who cannot afford the cost of journal subscriptions. Major open-access resources include journal portals, institutional repositories and e-books. Details about selected resources are outlined in Table 13.2.
Table 13.2: Open-Information Resources: Scholarly Literature
This table outlines the major open-access websites and resources where it is possible to access the full text of research papers, documents and books. The main types of resources found in this table include journal gateways, virtual libraries and repositories. In most cases, content from these websites can be reused, disseminated and copied without requesting permission from the creator.
|
Resource Platform |
Type |
Facts and Features |
|
Directory of Open Access Journals (DOAJ) |
Journal gateway |
|
|
HighWire Press |
Journal gateway |
|
|
Public Library of Science (PLOS) |
Journal gateway |
|
|
BioMed Central (BMC) |
Journal gateway |
|
|
Open J-Gate |
Journal gateway |
|
|
Biodiversity Heritage Library (BHL) |
Virtual library |
|
|
CGBooks on Google |
Virtual library |
|
|
OAISTER |
Virtual library |
|
|
Bielefeld Academic Search Engine (BASE) |
Search engine |
|
Social-media tools for researchers
On-line social-media platforms such as blogs, wikis and micro-blogging sites such as Twitter provide a quick, effective means of engaging with other researchers. They have rapidly changed the way science is communicated and disseminated in the last few years, allowing researchers to keep abreast of emerging trends and developments in their respective disciplines.
So what exactly are social media? They are “the use of web-based and mobile technologies to turn communication into interactive dialogue” (Wikipedia 2011a). Most social-media platforms encourage discussion, feedback and sharing of information. Tools such as Twitter, for example, allow a person participating in a conference to write short updates (called tweets) about what is being discussed in real time. The participant’s followers can keep abreast of the conference’s developments wherever they are located in the world, and they are able to respond and interact with the person providing the updates. This type of communication was unimaginable 16 years ago.
The uptake and use of these tools is increasing within the scientific and research community. Why are researchers increasingly using social-media tools? Gruzd and Staves (2011) and prior studies have highlighted three main benefits:
-
Researchers need to communicate with their peers, and social media allow them to do this regardless where they are located, at a low cost.
-
Social-media platforms provide researchers with the ability to create a community or network of like-minded scholars, which can facilitate and bring together people working on similar research.
-
Social media provide the opportunity to expand on ideas or research from the direct interaction between researchers and their readers.
In addition to Twitter, some of the more popular social-media platforms include wikis and blogs (see table 13.3 for specific links and more details about social-media platforms and tools). The wiki GRIN-Global is a good example of how researchers from different institutions in different locations in the field of plant genetic resources are able to collaborate and work together using social-media tools. Other social-media tools include forums (ScienceForums.Net) and platforms where one can upload and share scientific videos (SciVee), presentations (SlideShare) and images (Flickr). Only time will tell if these existing social-media platforms will be enhanced or even superseded by new technologies; however, the concept and value of “sharing ideas” is an integral part of human nature; consequently, it is difficult to think that social-media platforms will not continue to be part of our working and social domains for many years to come.
Table 13.3: Social Media Tools and Platforms
This table highlights social media websites that encourage scholarly discussion, feedback and sharing of information.
|
Resource name |
Coverage and Features |
|
Flickr |
|
|
Mixxt |
|
|
ResearchBlogging |
|
|
SciVee |
|
|
ScienceBlogs |
|
|
ScienceForums.net |
|
|
Slideshare/SlideBoom |
|
|
Twitter |
|
|
Wikis |
|
Bibliographical databases
Bibliographical databases are, and have always been, important tools for conducting research. With the advent of the internet, many of the bibliographic databases that were featured in the 1995 edition of the Technical Guidelines have remodelled themselves from standalone databases to web-based digital libraries. Often, these databases not only provide the full text to indexed and abstracted content, but also often provide the researcher with tools for analysis and are capable of carrying out federated searches across data silos. A federated search is an information-retrieval technology that allows the simultaneous search of multiple searchable resources. A user makes a single query request, which is distributed to the search engines participating in the federation. The federated search then aggregates the results that are received from the search engines for presentation to the user (Wikipedia 2011b).
These platforms are always in constant evolution and are consistently improving and extending content, search features and support tools. Table 13.4 provides a listing of the main bibliographic databases for agriculture and plant science, which will be useful to a researcher working in plant genetic resources.
Table 13.4: Agricultural Bibliographic Databases
This table highlights the main bibliographic databases that cover agriculture, PGR and the life sciences.
|
Resource name |
Coverage and Features |
|
AGRICOLA |
|
|
AGRIS |
|
|
CAB Abstracts/Plant Genetic Resources Abstracts (PGRA) |
|
|
FAO Corporate Document Repository |
|
|
Google Scholar |
|
|
ISI Web of Knowledge Access date: 07.07.2011 |
|
|
Mendeley Research Catalog |
|
|
MusaLit |
|
|
Science Citation Index (SCI) |
|
|
SciVerse |
|
|
Scirus |
|
|
Tropag and Rural |
|
|
World Wide Science.org |
|
Reference-management tools
With the huge amount of information and data available over the internet, researchers often struggle with how to manage the information they gather from databases and websites, as well as blogs and forums. Web-based reference-management systems can be the answer to this dilemma.
Reference-management software assists researchers and authors in managing the many bibliographic references they may have accumulated over time. The software is usually a database that allows for the creation of bibliographies and also acts as a personal online library. It has been around for many years, and with the advent of the internet, reference-management applications, like bibliographic databases, have evolved tremendously. From being databases installed on a stand-alone personal computer, these web-based platforms now have many features and tools. Apart from the traditional feature of generating bibliographies, today’s reference-management systems also possess social-networking elements, research catalogues, drag-and-drop features to reduce manual data inputting, and mobile applications for smart-phones and iPads. Some systems also allow users to save not only documents but also screenshots of web pages so that they have the ability to come back to the webpage at a later time. A growing number of reference-management systems are free over the web and can be of great benefit to researchers struggling to come to terms with the amount of information or data they have accumulated. Wikipedia (2011c) has a good article on reference management systems where it compares 29 systems (both free and proprietary). This information has been reproduced in part in table 13.5, which has a selection of the better known systems.
Table 13.5: Bibliographic Reference-Management Systems
Bibliographic reference-management systems are excellent tools for managing references and generating bibliographies and citations when writing research papers.
|
Software |
Developer |
Access |
Notes |
Operating systems |
Released |
|
Oversity Limited |
free |
|
Windows – Macintosh – Linux – BSD – Unix |
2004 |
|
|
Nature Publishing Group |
free |
|
Windows – Macintosh – Linux – BSD – Unix |
2004 |
|
|
Thomson Reuters |
cost |
has desktop and web account components |
Windows – Macintosh |
1998 |
|
|
Mendeley |
free & premium |
has desktop and web account components |
Windows – Macintosh – Linux |
2007 |
|
|
Thomson Reuters |
cost |
|
Windows |
1984 |
|
|
George Mason University |
free |
a Mozilla Firefox extension, so it can only be used with the Firefox browser |
Windows – Macintosh – Linux – BSD – Unix |
2006 |
Source: Wikipedia (2011c).
Future challenges/gaps/needs
In this chapter, we have looked at information tools and resources that can assist the researcher in the PGR domain. We have seen how a researcher can use many different platforms for resource discovery, and we have highlighted different formats/platforms that one can use to disseminate and generate content. All of these tools and resources will continue to evolve, change or be superseded by new technology, which ultimately means that researchers also need to keep abreast of how scientific information is generated and disseminated. Currently, a lot of focus has been given to mobile technology, where one can access information from smartphones or iPads and download applications that permit one to do an array of things, such as data analysis or creating tables. Tools such as these allow information to be generated and disseminated in a timely fashion; researchers do not need to wait until they are back at their desks. These tools have changed, and will continue to change, the way science is carried out, and it is to the researcher’s advantage to be aware of their availability and features.
As the scientific world moves more and more into the digital realm, one of the challenges researchers and their respective organizational libraries need to address is that a lot of scientific information is available only through subscription-based avenues. With the advent of digital information, a subscription often means that one is purchasing access to content but not the actual content, per se, as is the case with paper subscriptions, where one would put the journal on the shelf for as long as necessary. Company takeovers, for example, could affect access to digital information. Negotiations with publishers for perpetual access is necessary in order to retain access to content.
Another factor to contemplate is the gap in knowledge that exists between researchers from the South and those from the North. As Schisler (2011) states, “The digital divide is something we must not dismiss when considering the fact that anyone can now publish. The digital divide represents the socioeconomic difference among communities in their access to computers and the Internet. It is also about the required knowledge needed to use the hardware and software, the quality of these devices and connections, and the differences of literacy and technical skills between communities and countries…. When we think of the World Wide Web, as of today, it does not yet encompass the entire world. Many of the countries on the African continent, for example, have less than 5 Internet users per 100 inhabitants as opposed to the richer countries in the world that have over 50 Internet users per 100 inhabitants. We are seeing a small but decreasing gap in this divide, and this trend will continue.” Not everyone has the access to information that is taken for granted in many developed countries, and while the knowledge gap may be decreasing, it is still very evident in many developing countries. While addressing the question of how to overcome the complex issue of the digital divide is not the author’s intention, it is important to remind ourselves of the situation that is present today.
Conclusions
The last sixteen years has seen monumental changes in the way scholarly information is accessed, retrieved and re-used. As a result, the paradigm of scholarly publishing, communication and collaboration has changed dramatically since 1995 and it has provided the opportunity to bring the scientific community together in ways that before seemed impossible. This chapter has attempted to bring to the fore some of the most useful and pertinent resources for researchers working in the domain of plant genetic resources and to highlight some of the challenges, gaps and needs that could affect them or their respective organizations. The websites and research tools highlighted here will hopefully provide researchers with relevant and timely information, allow them to connect with their peers, and provide some respite from the “information overload” that one encounters when searching across the internet.
Back to list of chapters on collecting
References and further reading
Agichtein E, Gabrilovich E, Zha H. 2009. The social future of web search: modeling, exploiting, and searching collaboratively generated content. IEEE Data Engineering Bulletin 32(4):1–10. Available on-line (accessed 20 September 2011): http://sites.computer.org/debull/A09June/agichtein_ssm1.pdf .
Björk B-C, Welling P, Laakso M, Majlender P, Hedlund T, Guðnason G. 2010. Open access to the scientific journal literature: situation 2009. PLoS ONE 5(6): e11273/journal.pone.0011273. Available on-line (accessed 20 September 2011): www.plosone.org/article/info:doi/10.1371/journal.pone.0011273.
Gruzd A, Staves K. 2011. Trends in scholarly use of on-line social media. Academia.edu. Available on-line (accessed 20 September 2011): http://dal.academia.edu/AnatoliyGruzd/Papers/514997/Trends_in_scholarly_use_of_online_social_media.
Hajjem C, Harnad S, Gingras Y. 2005. Ten-year cross-disciplinary comparison of the growth of open access and how it increases research citation impact. IEEE Data Engineering Bulletin 28(4):39–47. Available on-line (accessed 20 September 2011): http://sites.computer.org/debull/A05dec/hajjem.pdf.
Harnad S, Brody T. 2004. Comparing the impact of open access (OA) vs. non-OA articles in the same journals. D-Lib Magazine 10(6). Available on-line (accessed 20 September 2011): www.dlib.org/dlib/june04/harnad/06harnad.html.
Schisler M. 2011. The future of publishing – introduction. Online Encyclopedia, Net Industries. Available on-line (accessed 18 August 2011): http://encyclopedia.jrank.org/articles/pages/1088/The-Future-of-Publishing.html.
StatCounter. 2011. Top 5 search engines from Oct to Dec 10. StatCounter Global Stats. Available on-line (accessed 29 July 2011): http://gs.statcounter.com/#browser-ww-monthly-201010-201012.
Suber P. 2011. Budapest open access initiative: frequently asked questions. Earlham College, Richmond, IN, USA. Available on-line (accessed 29 July 2011): www.earlham.edu/~peters/fos/boaifaq.htm#openaccess.
Swan A. 2010. The open access citation advantage: studies and results to date. School of Electronics & Computer Science, University of Southampton, UK. Available on-line (accessed 20 September 2011): http://eprints.ecs.soton.ac.uk/18516/1/Citation_advantage_paper.docx.
Swan A. n.d. Open access: a briefing paper for research managers and administrators. EnablingOpenScholarship, Liege, Belgium. Available on-line (accessed 20 September 2011): www.openscholarship.org/upload/docs/application/pdf/2009-01/briefing_paper_open_access.pdf.
Thomson Reuters. 2011. Journal Citation Reports 2010. Thomson Reuters, New York. See http://thomsonreuters.com/products_services/science/science_products/a-z/journal_citation_reports/.
Wikipedia. 2011a. Social media. Available on-line (accessed 19 September 2011): http://en.wikipedia.org/wiki/Social_media.
Wikipedia. 2011b. Federated search. Available on-line (accessed 25 August 2011): http://en.wikipedia.org/wiki/Federated_search.
Wikipedia. 2011c. Comparison of reference management software. Available on-line (accessed 28 July 2011: http://en.wikipedia.org/wiki/Comparison_of_reference_management_software.
More Articles...
- Chapter 10: Published sources of information on wild plant species
- Chapter 8: Sources of information on existing germplasm collections
- Chapter 19: Collecting and recording data in the field: media for data recording
- Chapter 22: Collecting vegetative material of forage grasses and legumes
- Chapter 24: Collecting in vitro for genetic resources conservation
- Chapter 15/16: Mapping the ecogeographic distribution of biodiversity and GIS tools for plant germplasm collectors
- Chapter 27: Collecting herbarium vouchers
- Chapter 26: Collecting symbiotic bacteria and fungi
- Chapter 7: Classifications of infraspecific variation in crop plants
- Chapter 21: Collecting vegetatively propagated crops (especially roots and tubers)
Subcategories
-
main
- Article Count:
- 1
-
Collecting
- Article Count:
- 31
-
Acquisition/Registration
- Article Count:
- 2
-
Sample processing
- Article Count:
- 1
-
Quality testing
What is quality testing?
The quality testing of seeds or plant materials assures that the materials to be conserved are in good conditions, i.e. can be grown again (viable) and are free of external contaminants (pests and diseases) and external genes (artificially produced genes). They are composed by three major aspects:
- Viability testing
- Plant health
- TransgenesThe quality of seed can be tested with a germination test
- Article Count:
- 5
-
Methods of conservation
- Article Count:
- 2
-
Cold storage
- Article Count:
- 1
-
Tissue culture
- Article Count:
- 1
-
Cryopreservation
- Article Count:
- 1
-
Molecular
- Article Count:
- 1
-
In field conservation
- Article Count:
- 1
-
Characterization
- Article Count:
- 1
-
Regeneration
What is Regeneration?
Regeneration is the renewal of germplasm accessions by sowing seeds or planting vegetative materials and harvesting the seeds or plant materials which will posses the same characteristics as the original population.
Germplasm regeneration is the most critical operation in genebank management, because it involves risks to the genetic integrity of germplasm accessions due to selection pressures, out-crossing, mechanical mixtures and other factors. The risk of genetic integrity loss is usually high when regenerating genetically heterogeneous germplasm accessions. Germplasm regeneration is also very expensive.Regeneration on fields
Why should germplasm be regenerated?
Germplasm is regenerated for the following purposes:
1. To increase the initial seeds or plant materials
In new collections or materials received as donations, the quantity of seeds or plant materials received by the genebank is often insufficient for direct conservation. Seeds or plant materials may also be of poor quality due to low viability or infection. All these materials require regeneration. Newly acquired germplasm of foreign origin may need to be initially regenerated under containment or in an isolation area under the supervision of the national phytosanitary authorities.
2. To replenishing seed stocks or plant materials in active and base collections
Increase seed stocks or plant materials of accessions that have:
- Low viability identified during periodic monitoring;
- Insufficient stocks for distribution or conservation.
Active collections should be regenerated from original seeds or plant materials in a base collection; this is particularly important for out-breeding species. Using seeds from an active collection for up to three regeneration cycles before returning to the original seeds or plant materials (base collection) is also acceptable (FAO/IPGRI 1994).
Base collections should normally be regenerated using the residual seed or plant materials from the same sample.How is it done?
If possible, regenerate germplasm in the ecological region of its origin. Alternatively, seek an environment that does not select some genotypes in preference to others in a population.
If no suitable site is found, seek collaboration with an institute that can provide a suitable site or regenerate in a controlled environment such as a growth room.
Examine the biotic environment in the context of prior information about the plants and past experience - an inappropriate biotic environment can be detrimental to plants, seed or propagation materials quality and the genetic integrity of an accession.Meeting special requirements
There may be special requirements for regeneration of accessions with special traits that breeders and researchers use frequently—such as high-yielding, pest-and disease-resistant accessions and genetic stocks — or if there are insufficient seeds for safety duplication and repatriation.
The following factors when regenerating germplasm accessions must be consider:- Suitability of environment to minimize natural selection;
- Special requirements, if any, to break dormancy and stimulate germination (such as scarification);
- Correct spacing for optimum seed set; and
- Breeding system of the plant and need for controlled pollination or isolation.Regeneration in a protected environment
When should it be done?
It should be done when either the quantity and/or the quality of a particular seed or plant material are not sufficient in a genebank.
The regeneration of accessions that have inadequate quality (low viability) should take priority over that of accessions with inadequate numbers of seeds or planting materials.
The regeneration of accessions in base collections should take priority over regenerating those in active collections.
- Article Count:
- 1
-
Dissemination
- Article Count:
- 1
-
Safety duplication
- Article Count:
- 1
-
Information/Documentation
- Article Count:
- 1
-
List of equipment and supplies
- Article Count:
- 1





.jpg)
.JPG)
.JPG)
.JPG)