CGKB News and events Management strategies
Nematodes - common bean
Contributors to this page: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck).
Root-knot nematode disease; Lesion nematode disease
Scientific name
Meloidogyne spp. and Pratylenchus spp.
Significance
Nematode infestations at high initial population densities cause significant yield losses. For example, yield losses may reach 10% to 80% with lesion nematodes, and 50% to 90% with root-knot nematodes. In addition, plant-parasitic nematodes, particularly the root-knot nematodes, are known to predispose many crop plants to various soil-borne microorganisms that induce root rot and wilt diseases.
Symptoms
Plants infected with nematodes do not necessarily exhibit characterisitic foliar symptoms. Severely infected plants may show chlorosis, stunting, necrosis of leaf margins and wilting during periods of moisture stress. Root-knot nematode infestation includes wilting, loss of vigour, yellowing and other symptoms similar to a lack of water or nutrients. Plants often wilt during the hottest part of the day, even with adequate soil moisture and leaves may turn yellow. Fewer and smaller leaves and fruits are produced, and plants heavily infested early in the season may die. Root-knot nematodes usually cause distinctive swellings,called galls, on the roots of affected plants. Infestations of these nematodes are fairly easy to recognize by digging up a few plants with symptoms, washing or gently tapping the soil from the roots, examining the roots for galls. The nematodes feed and develop within the galls, which may grow to as large as 1 inch in diameter on some plants but are usually much smaller. The water- and nutrient-conducting abilities of the roots are damaged by the formation of the galls. Galls may crack or split open, especially on the roots of vegetable plants, allowing the entry of soil-borne, disease-causing microorganisms. Root-knot nematode galls are true swellings and cannot be rubbed off the roots, as can the beneficial nitrogen-fixing nodules on the roots of legumes.
Hosts
Root-knot nematodes are obligate, endoparasites with a wide host range, including agronomic crops and weeds that belong to many plant families. Numerous plant parasitic nematodes are associates with roots and soils of bean and other plants throughout the world. As a genus, they are reported as parasites of over 3000 host plants and individual species often have a wide host range. Jensen et al. (1977) listed some 874 crop species as hosts of the seven or eight species commonly occurring in the western U.S. Root-knot nematodes may feed on the roots of grasses and certain legumes without causing galling.
Geographic distribution
Southeast Asia, South America and USA.
Biology and transmission
Root-knot nematodes (Meloidogyne spp and Pratylenchus spp) survive in soil as eggs and larvae. Length of survival in soil varies with the nematode species, stage of development, soil texture, soil moisture and soil aeration. Dissemination of nematodes among fields and growing regions can occur via irrigation water, vegetative plant parts and soil infested with eggs or larvae which adhere to farm tools, animals, or man. Depending upon the host and temperature, the entire life cycle may be completed in 17-57 days.
Detection/indexing method at CIAT
- Washing sedimentation test, visualization in stereomicroscopy and microscopy.
Treatment/control
To control root-knot nematodes, resistant plant varieties and chemicals are used. They are difficult to control and can be spread easily from garden to garden in soil (for example, on tools, boots, etc.) and plant parts. Growing crops antagonistic to nematodes such as Tagetes minuta L. (marigolds), Crotalaria spectabilis or Indigofera hirsute L. can reduce populations or even cause the elimination of root-knot nematodes. As with other cultural control methods, nematode populations will rapidly increase as soon as susceptible crops are grown. Crop rotation can reduce population levels of root-knot nematodes when beans are planted once every two or three years in rotation with non-hosts such as maize. Other cultural practices which reduce nematode populations include long fallow periods, deep plowing, weed control and, where practical, flooding for one or two weeks. However, the effectiveness of these organisms in the field and their economic commercial use are not encouraging. Chemical control of plant parasitic nematodes with nematicides is very effective and used widely on annual agronomic crops. However, use of nematicides is expensive for a crop like beans and requires care in handling, and often the use of special equipment for application.
References and further reading
Abawi GS, Crosier DC. 1985. Control of lesion nematodes on snap beans with fenamiphos. 1984. Fungi, Nemat. Test (USA) 40:94.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD, editors. 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources.
Schwartz HF, Pastor-Corrales MA, editors. Bean production problems in the tropics. Centro Internacional de Agricultura Tropical (CIAT). Cali, Colombia.
Best practices for safe transfer of common bean germplasm
Contributors to this page: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck).
|
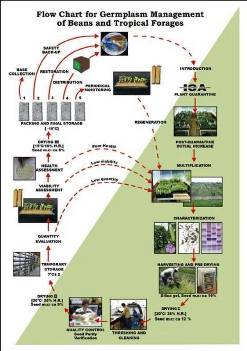
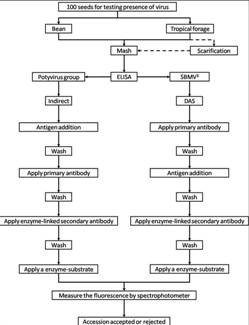
Flowchart 1.Germplasm management of Beans and Tropical Forages (Click to increase the size) |
The agreement between the CIAT and the International Treaty on Plant Genetic Resources for Food and Agriculture implies of the Genetic Resources Program (GRP) the conservation on behalf of the countries of 65,505 materials for 745 plant species of bean, cassava and tropical pastures as the biological patrimony of 141 countries. This responsibility of conservation is associated with the distribution of samples of this patrimony (517,916 samples distributed to 136 countries to the date) according to a regulation defined by the countries inside the Agreement, and phytosanitary procedure established by the Colombian Agricultural Institute.
The job of the GRP is to safeguard the genetic diversity of beans, cassava, forages, and their wild relatives through a mix of conservation methods, both in situ (in a natural outdoor habitat) and ex situ (in the controlled environment of a gene bank). Among the GRP's activities are research to improve conservation methods (including ways to minimize risks to the collections); screening germplasm for diseases and certifying it; duplicating materials of the collection; collecting or otherwise acquiring novel materials; recording passport, characterization and evaluation data for accessions of the collections (See Flowchart 1).
Phytosanitary risks are associated with international movement of germplasm, especially the inadvertent transport of pathogens and pests of quarantine significance. To minimize the phytosanitary risks associated with exchange germplasm and to ensure that it is free from pests and pathogens of quarantine significance; CIAT has implemented regulatory measures and safeguards to complement quarantine guidelines. The process, wich is supervised by the the Agricultural Colombian Institute (ICA), includes the following activities:
- Minimize the risk of accidental introduction of exotic pest and pathogens into Colombia;
- Inspect plant and facilities in screenhouses and grenhouses where the imported germplasm is being incresased;
- Inspect plants in fields and greenhouses where the germplasm intended for international export is produced; and
- Determine the seed health status of germplasm for international export.
The responsibility over the areas dealing with animal and plant health, with regard to international trade, has been bestowed upon the Agricultural Colombian Institute, within regulatory decree 1840 of 1994 “for which (ICA) has the mission of preventing the risk of the entry, spread, and establishment of exotic diseases, those of national sanitary concern, and of chemical risks, and protecting the sanitary quality of animals, plants and products that are exported, to minimize losses in animal-plant production and contribute to the security in foodstuffs”.
ICA has established quarantine procedures to regulate the introduction of plant germplasm and the issuing of phytosanitary certificates that accompany exports. ICA has a Plant Quarantine Station at an altitude of 2600 m (4o 42’ N latitude and 74o 12’W longitude) near Mosquera (Cundinamarca), about 15 km west of Santafé de Bogotá. ICA provides the regulatory mechanism for germplasm exchange following guidelines of the International Plant Protection Convention (IPPC). The recommendations of the IPPC were adopted by Colombian Congress in 1981 and implemented under decrees 501 of 1989.
In order to facilitate the importation and exportation, the ICA has developed the SISPAP, which may accessed through the ICA webpage in which the parties interested in importing and exporting plant products may become aware of the following via internet.
In Colombia, additional guidelines to reduce phytosanitary risks are in place. These safeguards are implemented according to the geographic origin and economic importance of the plant species concerned, and characteristic of potential pathogens and pests. Currently, ICA has a plant quarantine agreement with CIAT establishing which guidelines and safeguards are updated to facilitate germplasm exchange according to national and international requirements. An agreement was signed in 1981 between CIAT and ICA for quarantine procedures to regulate the introductions of germplasm into Colombia. The agreement permits the transit of seed through customs and quarantine stations according to the level of potential risk of introducing pests and diseases no yet reported in Colombia. High risk areas include Africa, Asia, and some European countries. The agreement covers not only crops for wich CIAT has a mandate but also other crops of economic importance to Colombia.
After clearance, materials pass through a step of multiplication in greenhouse stage, and the harvested seeds are subsequently planted in isolated fields. During these two steps, a phytosanitary follow-up is carried out. Plants showing any symptom of fungal, bacterial or viral disease are destroyed.
CIAT facilities for germplasm health testing are designed as a multifunctional laboratory to test seeds and tissues for fungi, bacteria, viruses, and occasionally nematodes and insects (See table below). The purpose of the Germplasm Health Laboratory (GHL) is to ensure that the designated germplasm is kept under the international phytosanitary standards for each crop commodity, and also to ensure that the germplasm distributed by GRP is free of diseases of quarantine importance (Listed in the table below)
The ICA Plant Quarantine Officer, stationed at CIAT, carries out field and greenhouses inspections and issues "ICA Phytosanitary Certificate" bases upon those inspections and results obtained by GHL. This document accompanies all out-going germplasm from Colombia (“decrees 1840 of 1994 articule 3”).
The germplasm leaving CIAT and the one used for exchange, conservation, and characterization in the GRP are multiplied in isolated fields under favorable ecological conditions with supervision by ICA quarantine officers. The GRP has seed multiplication sites in Popayan (Cauca) and Santander de Quilichao (Cauca). The harvested seeds are analyzed by GHL prior to shipment abroad or long-term conservation.
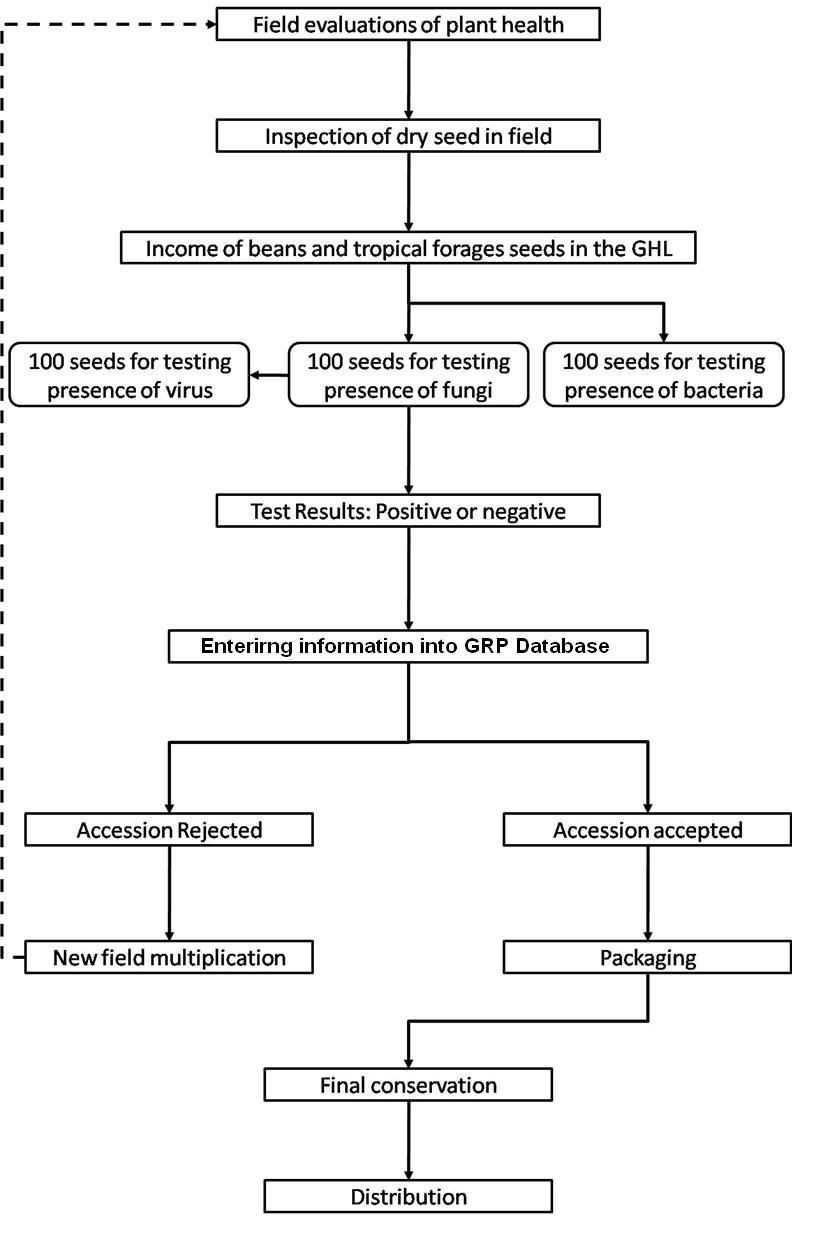
Seed health testing activities include:
- Reception, registration, sampling and storage of incoming material.
- Preparation of working samples for testing.
- Analysis.
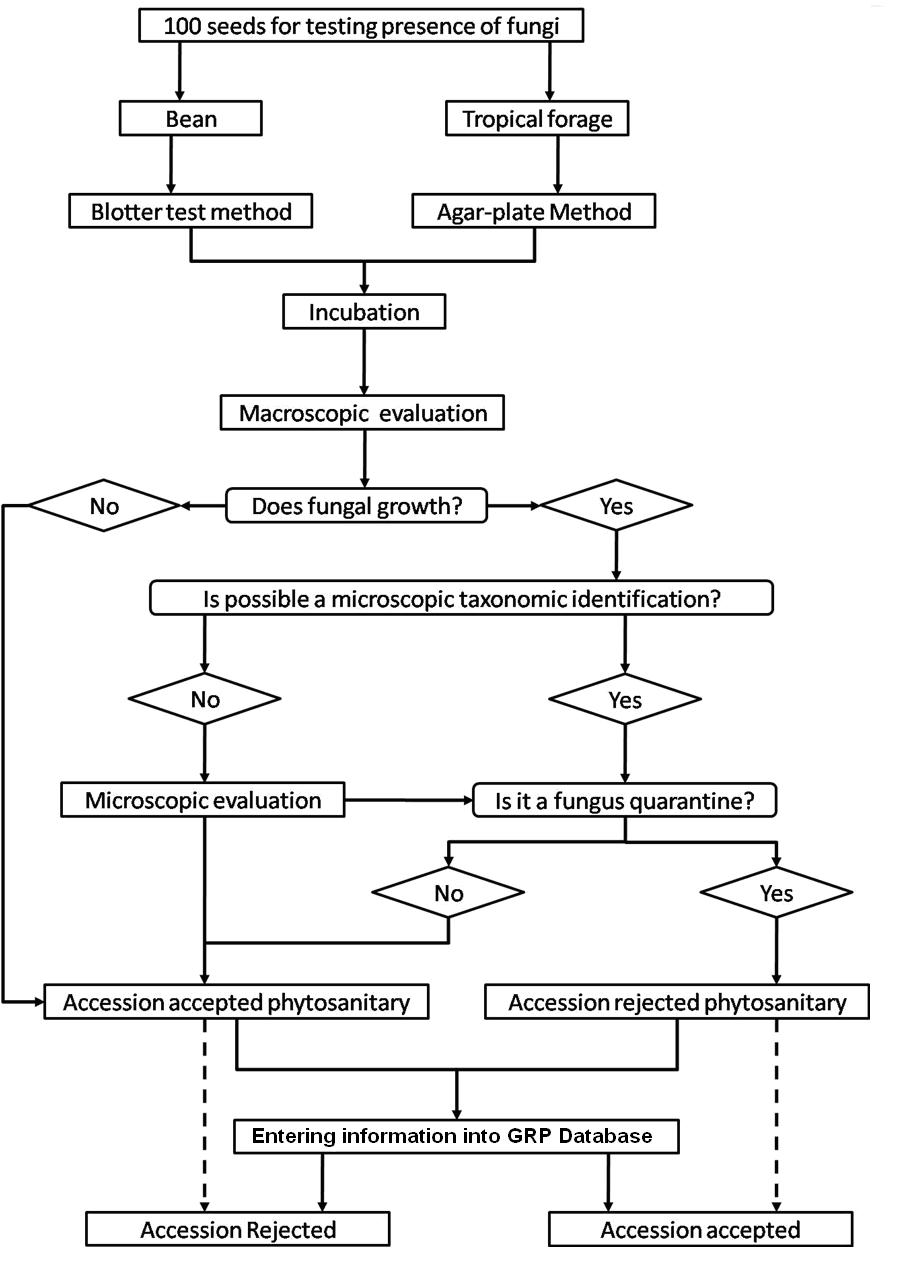
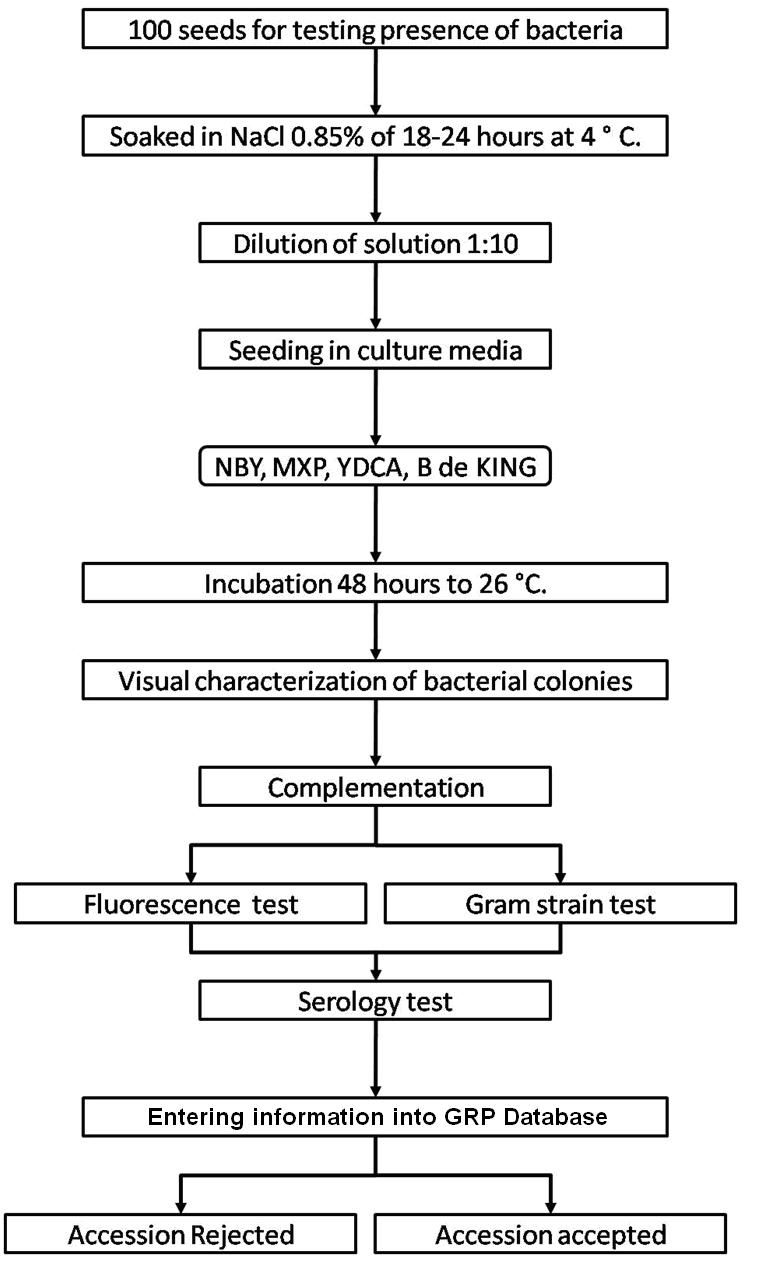
The seed health testing methods used at CIAT for beans and tropical pastures are summarized in The Handbook of Procedures of the Germplasm Health Laboratory (2.7 MB) (see also Flows charts 2, 3, 4, 5 below, for a quick reference).
To detect pathogens of quarantine significance, the GHL uses the methodologies recommended by CIAT pathologists and virologists (see table below). When a recipient country has additional requirements, the GHL carries out additional tests wherever possible to comply with the specific quarantine regulations of the recipient country. The GHL realizes additional researches in the management and characterization of pathogens of quarantine importance and in the standardization of new methodologies of diagnosis that are more effective and sensitive.
Seed health testing methods applied at GHL to detect pathogens of quarantine significance for common bean and related species
|
Viruses |
Seed Health testing methods |
| Peanut Mottle Virus (PeMOV) | Indirect ELISA, kit for Potyvirus group |
| Bean common mosaic virus (BCMV) | Indirect ELISA, kit for Potyvirus group |
| Bean southern mosaic virus (BSMV) | DAS-ELISA |
| Bacteria | Seed Health testing methods |
|
|
Seedling symptom test, Dilution plating test, MXP and YDCA semiselective culture medium, serology test with commercial kit |
| Pseudomonas syringae pv. phaseolicola | Seedling symptom test, Dilution plating test, King B semiselective culture medium, serology test with commercial kit |
| Curtobacterium flaccumfasciens | Seedling symptom test, Dilution plating test, NBY semiselective culture medium, serology test with commercial kit |
| Pseudomonas fluorescens Biotipo II | Seedling symptom test, Dilution plating test, King B semiselective culture medium, serology test with commercial kit |
| Fungi | Seed Health testing methods |
| Alternaria spp. | Seedling symptom test, Blotter Test and direct visualization in Stereomicroscopy and Microscopy. |
| Ascochyta phaseolorum Sacc. Current name: Phoma exigua var. exigua Sacc. 1879 | Seedling symptom test, Blotter Test and direct visualization in Stereomicroscopy and Microscopy. |
| Botrytis cinerea Pers. (Teleomorph. Sclerotinia fuckeliana (de Bary) Fuckel) | Seedling symptom test, Blotter Test and direct visualization in Stereomicroscopy and Microscopy. |
| Cercospora canescens Ellis & G. Martin | Seedling symptom test, Blotter Test and direct visualization in Stereomicroscopy and Microscopy. |
| Colletotrichum gloeosporioides (Penz.) Penz. And Sacc. | Seedling symptom test, Blotter Test and direct visualization in Stereomicroscopy and Microscopy. |
| Colletotrichum lindemuthianum (anamorph.), Gloromella cingulata (teleomorph.) | Seedling symptom test, Blotter Test and direct visualization in Stereomicroscopy and Microscopy. |
| Colletotrichum truncatum (Schwein.) Andrus | Seedling symptom test, Blotter Test and direct visualization in Stereomicroscopy and Microscopy. |
| Fusarium oxysporum Schltdl. Y F. solani f. sp. solani | Seedling symptom test, Blotter Test and direct visualization in Stereomicroscopy and Microscopy. |
| Macrophomina phaseolina (Tassi) Goid | Seedling symptom test, Blotter Test and direct visualization in Stereomicroscopy and Microscopy. |
| Phoma exigua Desmaz. var. diversispora (Bubak) Boerema | Seedling symptom test, Blotter Test and direct visualization in Stereomicroscopy and Microscopy. |
| Phomopsis subcircinata (anamorph.), Diaporthe phaseolorum (teleomorph) | Seedling symptom test, Blotter Test and direct visualization in Stereomicroscopy and Microscopy. |
| Phaeoisariopsis griseola (Sacc.) Ferraris | Seedling symptom test, Blotter Test and direct visualization in Stereomicroscopy and Microscopy. |
| Rhizoctonia solani Kühn (Teleomorph Thanatephorus cucumeris (frank) Donk.) | Seedling symptom test, Blotter Test and direct visualization in Stereomicroscopy and Microscopy. |
| Sclerotinia sclerotiorum | Seedling symptom test, Blotter Test and direct visualization in Stereomicroscopy and Microscopy. |
| Sclerotium rolfsii Sacc. (Teleomrph. Corticium rolfsii Curzi) | Seedling symptom test, Blotter Test and direct visualization in Stereomicroscopy and Microscopy. |
| Insects | Seed Health testing methods |
| Acantoscelides sp. | Direct visual inspection |
| Zabrotes spp. | Direct visual inspection |
| Nematodes | Seed Health testing methods |
| Meloidogyne spp. | Washing sedimentation test, Visualization in Stereomicroscopy and Microscopy |
| Pratylenchus spp. | Washing sedimentation test, Visualization in Stereomicroscopy and Microscopy |
References and further reading
Cuervo MI, Balcazar MS, Ramirez JL, Medina CA, Debouck D. 2009. Manual de operaciones laboratorio sanidad de germoplasma – unidad de recursos genéticos. GRP, CIAT, Colômbia. 72 pp. Available in Spanish (2.8 MB) and English (2.6 MB).
Viruses - common bean
Contributors to this page: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck).
|
Contents: |
Scientific name
Bean common mosaic virus (BCMV).
Other names
Mungbean mosaic virus (MBRV), Azuki bean mosaic virus, Bean mosaic virus, Bean western mosaic virus, Blackeye cowpea mosaic virus, Mungbean mosaic virus, Peanut stripe virus, Peanut mild mottle virus, Peanut chlorotic ring mottle virus, Sesame yellow mosaic virus.
Significance
The BCMV is the most widespread viral disease. It causes some of the most economically important diseases of legume crops around the world. Plant infection may reach 100% in fields and yield losses range from 6 to 98% depending upon the cultivar and time of infection. It is economically important throughout Africa, Europe, North America and Latin America.
Symptoms
The type of symptom produced is determined by the strain of BCMV, temperature and the host genotype. The BCMV may incite three types of symptoms: mosaic, systemic necrosis (black root), or local lesions or malformation. Symptoms associated with common mosaic include leaf rolling or blistering, light and dark green patches on the leaf (green mosaic), chlorotic vein banding, yellow mosaic and growth reduction. Mottling and malformation of the primary leaves is an indication that the primary infection occurred through seed. Cultivars which develop common mosaic may have distinct chlorotic or necrotic local lesions which are not associated with the vascular system. Systemically infected plants may have smaller and fewer pods and infected pods may sometimes be covered with small, dark green spots and mature later than uninfected pods. Black root is characterized by local necrotic lesions which extend into the veins causing systemic necrosis in the vascular system; this symptom only occurs in cultivars possessing the dominant resistance gene I. This necrosis can extend into the roots, stem and meristem and may result in plant death if the plant is infected at an early stage. Leaf distortion and blistering, dwarfing, downward curling of leaf margins, vascular necrosis, light and dark green mosaic, ring shaped and pin-point local lesions, distortion of flowers and buds.
Hosts
Natural hosts of BCMV are mainly restricted to Phaseolus spp., especially P. vulgaris . However, BCMV has been isolated naturally from other leguminous species including Phaseolus acutifolius, Phaseolus lunatus, Arachis hypogaea, Bauhinia purpurea, Cajanus cajan, Centrosema pubescens, Chenopodium quinoa, Cicer arietinum, Clitoria ternatea, Crotalaria incana, Crotalaria juncea, Crotalaria spectabilis, Cucumis sativus, Cyamopsis tetragonoloba, Glycine max, Lablab purpureus, Lupinus angustifolius, Lupinus luteus, Lupinus albus, Macroptilium atropurpureum, Macroptilium lathyroides, Medicago sativa, Melilotus alba, Pisum sativum, Rhynchosia minima, Senna sophera, Sena tora, Sesbania herbacea, Trifolium incarnatum, Trifolium pretense, Trifolium repens, Trifolium subterraneum, Trifolium hybridum, Vicia sativa, Vicia villosa, Vigna radiata, Vigna unguiculata, Vigna vexillata and Vigna subterranean.
Geographic distribution
The virus is probably distributed worldwide (in Phaseolus beans wherever they are grown).
Biology and transmission
Virus is of the genus Potyvirus in the family Potyviridae. Filamentous particles are about 750 nm long and 15 nm wide. Virus is transmitted by mechanical inoculation (sap), transmitted by seeds (up to 83% in Phaseolus vulgaris and from 7 to 22% in tepary bean, transmitted by pollen to seed. The virus is transmitted by arthropods, by insects of the order Hemiptera, family Aphididae; Acyrthosiphon pisum, Aphis craccivora, A. fabae, Myzus persicae and other species. The virus is transmitted in a non-persistent manner. Bean common mosaic is caused by two species of the genus Potyvirus: Bean common mosaic virus (BCMV) and Bean common mosaic necrosis virus (BCMNV).
Detection/indexing method in place at CIAT
- ELISA, kit for Potyvirus group.
Treatment/control
- Possible control measures for BCMV include planting healthy seed, improving cultural practices, controlling the vector and host plant resistance.
Procedure followed at the CGIAR Centres in case of positive test
- Reject accession and new regeneration seed process in field.
References of protocols at EPPO, NAPPO or other similar organization
ICTVdB Management (2006). 00.057.0.01.007. Bean common mosaic virus. In: ICTVdB - The Universal Virus Database, version 4. Büchen-Osmond, C. (Ed), Columbia University, New York, USA.
References and further reading
Alan B, Crabtree K, Dallwitz M, Gibbs A, Watson L, editors. 1996. Viruses of Plants. Description and Lists from the VIDE Database. CAB International, UK. 1484 pp.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD, editors. 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources.
Gad L, Thottappilly G, editors. 2003. Virus and Virus-like Diseases of Major Crops in Developing Countries. Kluwer Academic Publishers, Dordrecht. 800 pp.
Gálvez GE, Morales FJ. 1989. Aphid-transmitted viruses. In: Schwartz HF, Pastor-Corrales MA, editors. Bean production problems in the tropics. Centro Internacional de Agricultura Tropical (CIAT). Cali, Colombia. pp. 333-362.
Morales FJ. 2003. Common Bean, In: Loebenstein G, Thottappily G, editors. Virus and Virus-Like Diseases of Major Crops in Developing Countries. Kluver Academic Publisher, London, UK. pp. 425-445.
Morales FJ. 1979. Purification and serology of bean common mosaic virus. Turrialba 29(4):320-324.
Morales FJ, Bos L. 1988. Bean common mosaic virus. AAB Descriptions of Plant Viruses No. 337. Association of Applied Biologists, Wellesbourne.
Morales FJ, Castaño M. 2008. Enfermedades Virales del Frijol en America Latina. International Center for Tropical Agriculture, Virolgy Unit.
Morales FJ, Castaño M. 1986. Seed transmission characteristics of selected bean common mosaic virus strains in differential bean cultivars. Plant Dis. 71:51-53.
Olufemi Williams A, Mbiele AL, Nkouka N, editors. 1988. Virus Diseases of Plants in Africa. Organization of African Unity/Scientific, Technical & Research Commission (OAU/STRC), Technical Center for Agricultural & Rural Cooperation: Lagos Nigeria. 225 p.
 |
 |
|
|
Bean common mosaic (photos: URG-Virology Unit, CIAT) |
||
Scientific name
Peanut mottle virus (PeMoV).
Other names
Groundnut mottle virus, Peanut mild mosaic virus, Peanut severe mosaic virus.
Significance
Is considered to be economically important on a global scale.
Symptoms
Yellow vein clearing, crinkled leaves, blistered leaves, ring foliar lesions, variegation, deformed leaves, mottling with necrosis and mosaic chlorotic ringspot in leaves, mild leaf chlorosis and stunting.
Hosts
PeMoV is principally a disease of the peanut but occasionally it can affect fields of common bean in the surrounding areas of peanut production. Other host: Amaranthus retroflexus, Arachis hypogaea, Arachis pintoi, Beta vulgaris, Brassica rapa, Cajanus cajan, Chenopodium murale, Chenopodium quinoa, Chenopodium album, Citrullus lanatus, Crotalaria spectabilis, Cucumis sativus, Cyamopsis tetragonoloba, Glycine max, Lupinus angustifolius, Lupinus albus, Macroptilium lathyroides, Medicago sativa, Melilotus alba, Melilotus officinalis, Phaseolus acutifolius, Phaseolus lunatus, Pisum sativum, Senna bicapsularis, Senna obtusifolia, Senna occidentalis, Senna tora, Trifolium incarnatum, Trifolium pretense, Trifolium repens, Trifolium subterraneum, Trifolium hybridum, Vicia villosa, Vigna unguiculata and Vigna subterranean.
Geographic distribution
This virus affects bean in Africa, Australia, United States and Latin America.
Biology and transmission
The virus is of the Potyvirus genus in the Potyviridae family. The capsid is filamentous, flexuous with a clear modal length with a length of 740-750 nm. The virus is transmitted by mechanical inoculation; transmitted by seeds 0.02-2% in Arachis hypogaea; to 1% in Phaseolus vulgaris and to 0.008% in Vigna unguiculata (Demski et al., 1983), but not in Glycine max, Pisum sativum and Cassia obtusifolia. the virus is transmitted by arthropods, by insects of the order Hemiptera, family Aphididae; Aphis craccivora, A. gossypii, Hyperomyzus lactucae, Myzus persicae and Rhopalosiphum padi. The virus is transmitted in a non-persistent manner (A. craccivora can remain infective for 2 hours and M. persicae for 12 hours after acquisition (Paguio and Kuhn, 1974). Seed transmission in peanuts and beans, mottle and necrosis in peanut, bean and pea, Arachis glabrata resistant.
Detection/indexing method in place at CIAT
- ELISA, kit for Potyvirus group.
Treatment/control
- Prevent its spread to commercial seeds by destroying infected experimental peanut plantings.
- Roguing is another method of control but would not necessarily be reliable since symptoms are not always evident on recently infected plants.
- Aphid populations may be controlled by various registered insecticides.
Procedure followed in the case of positive test
- Rejection of accession and new regeneration seed process in field.
Protocols at other institutions
ICTVdB Management (2006). 00.057.0.01.049. Peanut mottle virus. In: ICTVdB - The Universal Virus Database, version 4. Büchen-Osmond, C. (Ed), Columbia University, New York, USA.
References and further reading
Alan B, Crabtree K, Dallwitz M, Gibbs A, Watson L, editors. 1996. Viruses of Plants. Description and Lists from the VIDE Database. CAB International, UK. 1484 pp.
Bijaisoradat M, Kuhn CWY, Benner CP. 1988. Disease reactions, resistance and viral antigen content in six legume species infected with eight isolates of peanut mottle virus. Plant Disease 72:1042-1046.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD, editors. 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources.
Gad L, Thottappilly G, editors. 2003. Virus and Virus-like Diseases of Major Crops in Developing Countries. Kluwer Academic Publishers, Dordrecht. 800 pp.
Kuhn CWY, Morales FJ. 2005. Peanut mottle. pp 79-80, in: Compendium of Bean Disease. Schwartz HF, Steadman JR, Hall R, Forster YRL. American Phytopathological Society, Segunda Edición, St. Pail, MN.
Marco S. 1986. Identification of Peanut mottle virus in Israel. Springer Netherlands. Volume 14, Number 3.
Morales FJ, Castaño M. 2008. Enfermedades Virales del Frijol en America Latina. International Center for Tropical Agriculture, Virolgy Unit.
Olufemi WA, Mbiele AL, Nkouka N, editors. 1988. Virus Diseases of Plants in Africa. Organization of African Unity/Scientific.
Technical & Research Commission (OAU/STRC), Technical Center for Agricultural & Rural Cooperation: Lagos Nigeria. 225 pp.
Thomas JE, Redden RJ, Usher T. 1990. Screening for resistance to peanut mottle virus in accessions and breeding lines of Phaseolus vulgaris. pp. 163-164, in: Bean Improvement Cooperative 1990 Annual Report.
Scientific name
Bean southern mosaic virus (BSMV)
Other scietnific names
Southern bean mosaic virus 1
Significance
BSMV is undoubtedly the most widely distributed of the beetle-borne viruses which infects beans. It is considered to be economically important on a global sacale. The virus can reduce bean yields as much as 83 to 94 % reducing the amount and weight of seed produced by infected plants.
Symptoms
In Phaseolus it can induce diverse symptoms such as mosaic, or mottle, rugosity, epinasty, vein yellowing, stunting, and necrotic local lesion, depending on the variety inoculated.
 BSMV (photo: Virology Unit, CIAT) |
Hosts
The virus has a host range restricted to legumes. Casia tora, Cicer arietinum, Cyamopsis tetragonoloba, Glycine max, Lupinus albus, Melilotus albus, Phaseolus acutifolius, P. lunatus, P. vulgaris, Pisum sativum, Vigna mungo, V. radiata, V. subterranea, V. unguiculata and Vigna unguiculata ssp. Sesquipedalis.
Geographic Distribution
The virus was first observed in southern United States and now is present in all the main bean producing nations of the world. The virus spreads in Africa, North America, South and Central Americas, France and Spain.
Biology and transmission
SBMV is a type member of the sobemovirus group which characteristically has isometric particles 28 to 30 nm in diameter and contains one molecule of positive-sense single-stranded RNA. The virus is transmitted by a vector. The virus is transmitted by sap, mechanical inoculation, grafting, seeds 3-7% in V. unguiculata cv.; it is transmitted by pollen to seed and transmitted by pollen to the pollinated plant. The virus is seed-borne and can be carried both in the embryo and as a contaminant on the seed coat. This virus, however, becomes inactivated upon the dehydration or storage of contaminated seeds. Secondary transmission occurs naturally by several species of chrysomelid beetles such as Cerotoma facialis, C. trifurcate and Epilachna varivestis. The virus is transmitted in a semi-persistent manner.
Detection/indexing method in place at CIAT
- ELISA with commercial kit.
Treatment/control
- Virus free seed, grown in areas where the virus does not occur, should be used.
- Insecticides that reduce beetle population should be helpful.
- BSMV is best controlled by planting resistant cultivars.
Procedure followed in the case of positive test
- Reject accession and new regeneration seed process in field.
Protocols at other organizations
ICTVdB Management (2006). 00.067.0.01.001. Southern bean mosaic virus. In: ICTVdB - The Universal Virus Database, version 4. Büchen-Osmond, C. (Ed), Columbia University, New York, USA.
References and further reading
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD, editors. 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Gad L, Thottappilly G, editors. 2003. Virus and Virus-like Diseases of Major Crops in Developing Countries. Kluwer Academic Publishers, Dordrecht. 800 pp.
Gergerich RC, Morales FJ. 2005. Bean southern mosaic. Pp 71-72. In: Schwartz HF, Steadman JR, Hall R, Forster YRL, editors.Compendium of Bean Disease. American Phytopathological Society, Second edition, St. Paul, MN.
Jayasinghe U. 1982. Chlorotic mottle of bean. PhD Thesis, Agric. Univ. Wageningen, the Netherlands. 156 pp.
McDonald JG, Hamilton RI. 1972. Distribution of Southern bean mosaic virus in the seed of Phaseolus vulgaris. Phytopathology 62:387-389.
Morales FJ, Castaño M. 1985. Effect of a Colombian isolate of bean southern mosaic virus on selected yield components of Phaseolus vulgaris. Plant Diseases 69:803-804.
Morales FJ, Castaño M. 2008. Enfermedades Virales del Frijol en America Latina. Interantional Center for Tropical Agriculture, Virolgy Unit.
National Bureau of Plant Genetic Resources. 1994. Checklist on Seed Transmitted Viruses: Leguminous Hosts. National Bureau of Plant Genetic Resources (NBPGR), India. 14 pp.
Shepherd RJ, Fulton RW. 1962. Identity of a seed-borne virus of cowpea. Phytopathology 52:489-493.
Tremaine JH, Hamilton RI. 1983. Southern bean mosaic virus. CMI/AABDescriptions of Plant Viruses No. 274. Commonwealth Agricultural Bureaux, Slough.
Insects - common bean
Contributors to this page: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck).
|
Contents: |
Scientific names
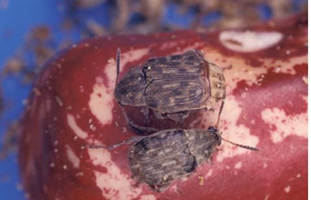
Acantoscelides sp, A. obtectus, A. obvelatus and A. argillaceu
Significance
No precise information on losses in stored beans by bruchids is available. However, farm storage for six months is accompanied by about 40% loss in weight with as much as 80% of the seeds being infested and unfit for human consumption. Losses vary between 7% in Colombia to 73% in Kenya.
The Bruchidae are adapted to attack the mature seeds of legumes, and those which attack food legumes demonstrate a certain degree of specialization to different legume species. The larva penetrates the seed and subsequent development takes place entirely within the seed cotyledons. The larva pupates within the seed but before doing so prepares the point of its eventual escape from the seed by chewing away a circular escape tunnel through the cotyledon leaving intact only the testa at the outside. This area of undermined testa can be seen clearly as a grey ‘window’.
Hosts
It is a widely distributed pest of storage beans.
Geographic distribution
Cosmpolitan.
Biology and transmission
Bean weevils are generally compact and oval in shape, with small heads somewhat bent under. Sizes range to 1mm, up to 22mm for some tropical species. Colours are usually black or brown often with mottled patterns. Although their mandible may be elongate they do not have long snouts. Adults deposit eggs on seeds, then the larvae chew their way into the seed. When ready to pupate, the larvae typically cut an exit hole, and then return to their feeding chamber. Adult weevils have a habit of feigning death and dropping from a plant when disturbed. Typically they infest various kinds of beans, living for most of their lives inside a single seed. Acantoscelides obtectus and Acantascelides obvelatus – develop in Phaseolus vulgaris; Acantascelides argillaceus – develop in Phaseolus lunatus. A. obtectus predominates in cooler areas. Eggs are laid on stored beans or in cracks of growing pods in the field. The larvae tunnel into the seeds to feed. Adult weevils are short-lived and do little feeding. A. obtectus is a better competitor than Zabrotes subfasciatus at lower temperatures and will eventually predominate under these conditions. A. obtectus females do not glue eggs to the test but scatter them among stored seeds or infest beans in the field by ovipositing on growing pods. The newly hatched larvae will later penetrate the seed. A. obtectus live 14 days and lay an average of 45 eggs.
Detection/indexing method at CIAT
- Direct visual inspection.
Treatment/control
Chemical control of harmful species from the Coleoprera Order is acceptable only in certain very limited situations, and unacceptable in the majority of cases for the residual toxicity. The following controls are recommended: Preventive control measures: overall cleaning measures, specific preventive measures, physical treatments; preventive measures applied when introducing the products into the storehouse; periodical supervision measures; curative, non-polluting measures (dehydration powders, means of entheroleter, means of pheromone traps, use of microwave electromagnetic generators, use of strong electric fields and “corona” discharges in alternative current, use of vegetal insecticides.
Procedure followed in case of positive test
Not noted.
References and further reading
Porca M, Ghizdavu I, Oltean I, Bunescu H. 2003. Control of the coleopteres in stored agricultural products by not-chemical methods. Journal of Central European Agriculture (online), 218 Volume 4 (2003) No3.
 |
 |
|
|
Acanthoscelides obtectus (photos: Beans Entomology, CIAT and Miroslav Deml, Biolib images) |
||
Scientific name
Zabrotes spp. Zabrotes subfasciatus (Boheman)
Other names
Zabrotes pectoralis (Sharp), Spermophagus subfasciatus Boheman, Spermophagus musculus Boheman, Spermophagus pectoralis Sharp, Spermophagus semifasciatus Boheman.
Significance
It is the most important pest of storage beans in warmer regions.
Symptoms
Not noted.
Hosts
Beans, cowpea and vigna unguiculata. All the known hosts of Zabrotes are in the Fabaceae with a questionable record in the Bixaceae.
Geographic distribution
Cosmopolitan. It is a tropical species and is found predominantly in warmer areas.
Biology and transmission
Zabrotes subfasciatus attaches the egg to the seed. After hatching, the young larvae bore through their egg shell and the seed coat in one process. Zabrotes subfasciatus does not attack in the field. Adults exhibit strong sexual dimorphism. Females are large and have four characteristic cream-coloured spots on the elytra. The male is entirely brown. At 28oC and 75%-80% r.h. Females lay an average of 36 eggs and live 13 days. The egg stage lasts five to six days, larval development takes 14 days, and the pupal stage takes six to seven days.
Detection/indexing method at CIAT
- Direct visual inspection.
Treatment/Control
Chemical control of harmful species from the Coleoprera Order is acceptable only in certain very limited situations, and unacceptable in the majority of cases for the residual toxicity. The following controls are recommended: Preventive control measures: overall cleaning measures, specific preventive measures, physical treatments; preventive measures applied when introducing the products into the storehouse; periodical supervision measures; curative, non-polluting measures (dehydration powders, means of entheroleter, means of pheromone traps, use of microwave electromagnetic generators, use of strong electric fields and “corona” discharges in alternative current, use of vegetal insecticides.
References and further reading
Porca M, Ghizdavu I, Oltean I, Bunescu H. 2003. Control of the coleopteres in stored agricultural products by not-chemical methods. Journal of Central European Agriculture (online), 218 Volume 4 (2003) No3.
 |
 |
|
|
Zabrotes subfasciatus (photos: Beans Entomology, CIAT and htpp://entmuseum9.ucr.edu/ENT133/ebeling/ebeling7.html#sitophilus granarius) |
||
More Articles...
- Weeds (common bean)
- Safe transfer of forage legume germplasm
- Import/export of forage legume germplasm
- Guidelines for the safe transfer of forage legume germplasm
- Bacteria - forage legume
- Fungi - forage legume
- Nematodes - forage legume
- Best practices for the safe transfer of forage legume germplasm
- Viruses - forage legume
- Insects - forage legume
Subcategories
-
main
- Article Count:
- 11
-
Stog
- Article Count:
- 2
-
Stog-rice
- Article Count:
- 7
-
Stog-sorghum
- Article Count:
- 11
-
Stog-common-bean
- Article Count:
- 10
-
stog-forage-legume
- Article Count:
- 10
-
stog-forage-grass
- Article Count:
- 11
-
stog-maize
- Article Count:
- 9
-
stog-chickpea
- Article Count:
- 10
-
stog-millets
- Article Count:
- 12
-
stog-barley
- Article Count:
- 10
-
stog-groundnut
- Article Count:
- 9
-
stog-pigeon-pea
- Article Count:
- 8
-
stog-wheat
- Article Count:
- 10
-
stog-lentil
- Article Count:
- 9
-
stog-cowpea
- Article Count:
- 10
-
stog-faba-bean
- Article Count:
- 9
-
risk management
- Article Count:
- 4
-
decision support tool
- Article Count:
- 3
-
stog-clonal
- Article Count:
- 23
-
developing strategies
- Article Count:
- 4