CGKB News and events Banana
|
Importance and origin Banana (Musa spp.) is one of the most ancient fruit crops known and used by man. It originated in South-East Asia and was first domesticated some 7000 years ago. Today it is grown in every humid tropical and many sub-tropical regions. It is the fourth most valuable food crop after rice, wheat and maize. The fruit crop provides a staple food source for 400 million people, most important in East–Africa. About 95 million metric tons of bananas are harvested annually around the world, 30% of these being plantains. About 90% of the total production takes place on small-scale farms and it is used for home consumption or in domestic markets. The remaining 10% (dessert bananas) are mostly produced in Latin-America and Caribbean and commercialized in world trade.
Production of banana in the world
|
|
|
How is it consumed? For many people in the tropics, bananas are an essential component in their daily diet.
Bananas are essential for many people in the tropics
|
|
|
Which types exist? Bananas evolved from inter-and intraspecific hybridization between two diploid wild species of the genus Musa, sp. acuminata (AA) and sp. balbisiana (BB), the only species that set seeds. These crosses produced edible cultivars (all female sterile with various levels of male sterility) currently cultivated, with the following genomic configuration:
|
|
|
Further reading: Simmonds and Shepherd, 1955 |
Banana
Contact person for Banana: Bart Panis, Bioversity, Belgium
Contributors to this page: Bioversity International, France (Nicolas Roux, Anne Vezina, Max Ruas, Stephanie Channeliere), Bioversity International, Belgium (Bart Panis, Ines Van den Houwe), Bioversity International, Ethiopia (Michael Bolton, Alexandra Jorge), CIRAD, France (Jean Pierre Horry, Tomekpe Kodjo), Institute of Experimental Botany (Jaroslav Dolezel), IITA, Nigeria (Dominique Dumet).
Compilation of best practices
Information on current practices for genebank management of banana was gathered from available literature and websites, as well as from current practices and accumulated experience from laboratories and genebanks holding major banana collections (Bioversity International, IITA, CIRAD and EMBRAPA genebanks). Information on in vitro and cryopreservation was provided by Bioversity International, complemented with extracts from Part I (Refinement & Standardization of Storage Procedures for Clonal Crops) and Part II (Status of In vitro Conservation Technologies for: Andean Root & Tuber crops, Cassava, Musa, Potato, Sweetpotato & Yam) of a three-part document prepared with the consultants E. Benson and K. Harding, as well as the clonal crop experts D. Debouck, D. Dumet, R. Escobar, G. Mafla, B. Panis, A. Panta, D. Tay, I. Van den Houwe and N. Roux. Information was then edited and uploaded onto this website, complemented with relevant photos and revised and validated by the crop experts.
Importance and origin
|
|
Banana (Musa spp.) is one of the most ancient fruit crops known to and used by man. It originated in Southeast Asia and the Pacific region and is believed to have been first domesticated more than 7000 years ago. East and Central Africa are secondary centres of domestication.
About 150 million tonnes of bananas are harvested annually throughout the tropics (FAOSTAT 2011), but only 15% or so (mainly dessert types) of that production is traded internationally. Most bananas are grown for home consumption or for sale in domestic or regional markets. The cooking types are especially important for food security. East Africa has the highest consumption rates, with up to 1 kg a day per person.
Types
Wild bananas are native to the tropical and sub-tropical forests of Asia and Oceania. They produce inedible fruits that are full of seeds and, like humans, they are diploid, that is they have two complete sets of chromosomes.
More than 50 species of bananas are believed to exist, but two species, Musa acuminata and Musa balbisiana, are better known for their role in the domestication of most types of bananas (a distinct group of cultivars, Fe'i bananas, developed independently in the Pacific region). Overall, there are believed to be approximately 1000 varieties of bananas.
A nomenclature system based on morphological characters was developed to group varieties according to the contribution of their wild ancestors, designated by the letter A for acuminata and B for balbisiana. For example, the AA genome group comprises varieties that have two acuminata genomes, while the AAB genome group is for varieties that have one balbisiana genome and two acuminata genomes. Genome groups are further divided into subgroups, which represent varieties that are closely related to each other, or appear to be related.
The main subgroups of dessert bananas are Sucrier (AA), Ney Poovan (AB), Cavendish (AAA), Gros Michel (AAA), Mysore (AAB), Pome (AAB) and Silk (AAB). The main subgroups of cooking bananas are East African Highland Bananas (AAA), Plantain (AAB), Maoli-Popoulu (AAB), Bluggoe (ABB), Saba (ABB) and Pisang Awak (ABB). With a few exceptions, diploid varieties are now rare, having been replaced by the more vigorous and productive triploid varieties.
Cultivar diversity images (Source: Musarama)
|
Products include chips, dried sweet bananas, beer, wine, juice, baskets and mats from banana fibre, among many others (photos 1,4,6: Bioversity; 2: Z-Corcuerra Food Products; 3: Taless Dry Foods; 5: CREED; 7: La Tunosa Cooperative of Rural Women; |
Utilization
The banana is widely known as a sweet fruit to be eaten raw, but many varieties are fried, roasted, juiced, dried or made into chips. The fruit can also be brewed into alcoholic beverages or made into flour by drying and grinding the dried fruits. In certain places, the male inflorescence is eaten as a vegetable.
Other parts of the plant are also used. Throughout the tropics, people exploit the large, smooth banana leaf for wrapping food. Banana leaves are also used for thatching, as umbrellas and plates. Products made from the different parts of the plant are also a much needed source of income. Banana fibre extracted from the stem appears in paper, bank notes, ropes, clothes and baskets. Anything left over can be used as animal feed.
References and further reading
For more information about banana, visit the banana knowledge compendium on the website of the global banana R4D community ProMusa: www.promusa.org/musapedia
For more information about the global network for conservation and use of Musa, visit the MusaNet website: www.musanet.org
For more literature on banana, visit the bibliographic database Musalit: www.musalit.org
Denham T, De Langhe E, Vrydaghs L. 2009. Special issue: history of banana domestication. Ethnobotany Research and Applications 7:163-164. Articles included in this special issue available from http://lib-ojs3.lib.sfu.ca:8114/index.php/era/issue/view/25.
INIBAP. 2006. Global Conservation Strategy for Musa (Banana and Plantain). Available from: www.croptrust.org/documents/cropstrategies/banana.pdf. Accessed: 23 March 2010.
Mbida MC, Doutrelepont H, Vrydaghs L, Swennen R, Swennen RJ, Beeckman H, De Langhe E, de Maret P. 2005. The initial history of bananas in Africa. A reply to Jan Vansina, Azania, 2003. Azania XL. The British Institute in Eastern Africa. pp. 128-135.
Mbida MC, Doutrelepont H, Vrydaghs L, Swennen R, Swennen RJ, Beeckman H, De Langhe E, de Maret P. 2004. Yes, there were bananas in Cameroon more than 2000 years ago. Infomusa 13 (1):40-42. Available here.
Ploetz RC, Kepler AK, Daniells J, Nelson SC 2007. Banana and plantain—an overview with emphasis on Pacific island cultivars. Available from: www.agroforestry.net/tti/Banana-plantain-overview.pdf. Date accessed: 23 March 2010.
Simmonds NW. 1962. The evolution of the bananas. Tropical Agricultural Series. Longman Scientific and Technical, UK. 170 pp.
Simmonds NW, Shepherd K. 1955. The taxonomy and origins of the cultivated bananas. Journal of the Linnean Society (Bot.), 55 (359):302-312. Abstract available from: www3.interscience.wiley.com/journal/119777761/abstract?CRETRY=1&SRETRY=0. Date accessed: 23 March 2010.
Stover RH, Simmonds NW. 1987. Bananas. Tropical Agricultural Series. Longman Scientific and Technical, UK. 468 pp.
background docs banana
ActaHort_1998_490
Conservation of Plant Genetic_
INIBAP International Network for the improvement of Banana
Management of Banana_2006_
Minimum set of photos for accession identification
Mussaspp
Mussa Germplasm Distribution
Organicmanuel04_es
PlantCellTissueOrganCulture
tg8_en
Registration of banana genetic resources
Contributors to this page: Bioversity International, Belgium (Ines Van den Houwe), Bioversity International, France (Max Ruas, Nicolas Roux); IITA, Nigeria (Dominique Dumet, Badara Gueye).
Verifying accompanying documentation
The following is a list of required documentation to be sent with the new materials.
- Import permit issued by the recipient country.
- Phytosanitary certificate issued by the official phytosanitary plant authority of the donor country.
- A list of accessions being shipped.
Verifying the consignment
The following is the required inspection procedure for plants to determine any plant health problems.
- Visual inspection for fungi and bacteria.
- Virus indexing of materials (see plant health diagnosis for more details).
- If any pathogens are detected, materials should be destroyed.
- If germplasm is rare, the pathogens should be eliminated before introducing the materials into the genebank.
Assigning numbers
New accessions are usually received as small samples of in vitro cultures, classical vegetative propagation material (young suckers) or as seeds.
- Upon introduction of the new accessions into the genebank, a ‘temporary identifier’ must be assigned to each individual sample of the new accession until it is decided which sample, or set of derived sub-samples, will be included in the genebank and registered officially.
- This temporary identifier usually consists of the accession's name and code, as provided by the donor, and the number of replicate samples e.g. a serial number (1, 2, or I, II, III) assigned at the genebank.
- Tissue cultures derived from one single sample (shoot tip) should be tested aseptic (i.e. free of contaminating fungi and bacteria).
- A minimum number (7) of vigorously growing tissue cultures derived from the selected sample should be established.
- The official registration involves the allocation of a permanent and unique identifier code.
If the conditions described above cannot be met, then a temporary accession number must be assigned and plantlets must go through the necessary disease cleaning or multiplication process until they can be assigned a permanent number.
Procedures for disease testing
Procedures for disease testing at ITC include sending a set of five cultures to Virus Indexing Centres at CIRAD, France, QDPI, Australia or PPRI, South Africa, where the materials are tested for the presence of the five major banana viruses:
- Cucumber mosaic cucumovirus (CMV).
- Banana bunchy top virus (BBTV).
- Banana streak virus (BSV).
- Banana mild mosaic virus (BanMMV).
- Banana bract mosaic virus (BBrMV).
A step by step guide to registration procedures is available by clicking on this link.
Extra samples
A separate subset of material can be kept after registration, to be regenerated under greenhouse conditions, for harvesting leaf samples that can be processed for DNA/lyophilized leaf bank. These banked leaf materials can serve as a reference for molecular identification for the germplasm stored in the active and base collections and samples can be made available to users for research in gene discovery and function, marker development and detailed genotypic characterization.
Recording information during registration
New materials - Introduction phase
Newly introduced meristems or nodal cuttings should be processed in batches. For each batch, a series of information should be recorded in a table with the following fields (at IITA):
- Batch number.
- Accession number.
- Date of in vitro introduction.
- Number of explants introduced.
- Contamination.
- Necrosis.
- Operator.
- Numbers of seedlings send to multiplication 1.
- Contamination while in multiplication 1.
- Necrosis while in multiplication 1.
- Number of seedlings sent to the genebank.
Musa Germplasm Information Systems (MGIS)
In 1997, INIBAP laid the basis for a global information system for Musa through the release of MGIS. The aim of the system was to enhance knowledge on Musa diversity, to help rationalize conservation and to improve the use of banana genetic resources though a facilitated access to comprehensive information.
In 2005, the MGIS database contained key information, including passport data, botanical classification, morpho-taxonomic descriptors and characteristics such as agronomic traits, disease resistance, stress tolerance, biochemical or molecular genetic markers, and plant photographs, as well as GIS information on 5188 accessions managed in 18 banana collections around the world, making it the most extensive source of information on banana genetic resources.
The database is publicly accessible through the internet here. This global database can be queried on the identity, origin, characteristics and distribution of the individual accessions in the collections. This allows curators of the participant institutions worldwide to share and compare their data. The database is also particularly helpful for various germplasm users, namely breeders, researchers and farmer communities, to locate alternative sources of banana germplasm and identify the most appropriate accessions with particular traits of interest.
Homepage of the MGIS website (click on the picture to go to the MGIS homepage).
References and further reading
de Vicente C, Fulton T. 2003. Using molecular marker technology in studies on plant genetic diversity: Learning module Vol 1. IPGRI, Rome, Italy. View learning module here. Date accessed: 23 March 2010.
Engels JMM, Visser L, editors. 2003. A guide to effective management of germplasm collections. IPGRI Handbooks for Genebanks No. 6. IPGRI, Rome, Italy. Available in English (1.4 MB) and Spanish. (1.5 MB)
International Union for the Protection of New Plant Varieties (UPOV). 1991. International Convention for the Protection of New Varieties of Plants. UPOV, Geneva. Available upon request in English, French, German and Spanish from: www.upov.int/en/publications/index.html. Date accessed: 23 March 2010.
Rao NK, Hanson J, Dulloo ME, Ghosh K, Nowel D, Larinde M. 2006. Manual of seed handling in genebanks. Handbooks for Genebanks No. 8. Bioversity International, Rome, Italy. Available in English (1.5 MB), Spanish (1.4 MB) and French. (1.9 MB)
Van den Houwe I, Panis B, Arnaud E, Markham R, Swennen R. 2006. The management of banana (Musa spp.) genetic resources at the IPGRI/INIBAP genebank: the conservation and documentation status. In: Segers H, Desmet P, Baus E, editors. Tropical biodiversity: science, data, conservation. Meeting: 3rd GBIF Science Symposium, Brussels, 18-19 April 2005. pp. 141-150. Available here. (8 MB)
Conservation of banana genetic resources
Contributors to this page: Bioversity International, France (Nicolas Roux, Anne Vezina, Max Ruas, Stéphanie Channeliere), Bioversity International, Belgium (Bart Panis, Ines Van den Houwe), Bioversity International, Ethiopia (Michael Bolton, Alexandra Jorge), CIRAD, France (Jean-Pierre Horry), Institute of Experimental Botany (Jaroslav Dolezel), IITA, Nigeria (Dominique Dumet, Badara Gueye).
Importance of banana conservation
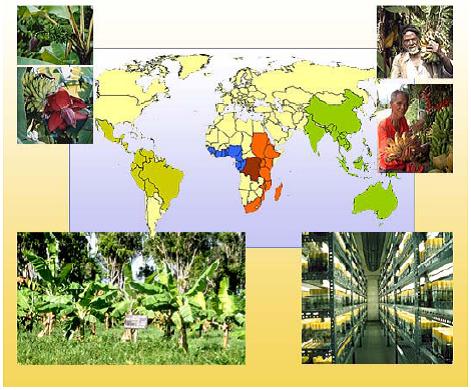 |
|
People in different parts of the world work together to conserve banana diversity |
Genetic improvement of varieties presents a potentially cost-effective mechanism to address current constraints in smallholder production, providing high-performing varieties that can adapt to diverse environments. These potential improvements are drawn from sources of resistance from wild and edible genotypes. However, new wild species and varieties are inadequately represented in collections. Threats posed by habitat destruction and replacement or loss of traditional cultivars intensify the urgency for collection and conservation efforts worldwide (INIBAP, 2006).
Recent banana projects have demonstrated a growing demand for increased diversity of cultivars, improved varieties not only within the research and breeding community but also among smallholder farmers and formal market systems. Supplying banana producers with a wider range of diversity can potentially broaden their livelihood options and can also enable family nutrition to be diversified (INIBAP, 2006).
Conservation of banana genetic resources is together with the other roots and tubers also subject of theme 1 “Conserving and accessing genetic resources” of the CGIAR Research Program on roots, rubers and bananas (CRP-RTB). Theme 1’s overall objective is to build upon the existing competencies of the four CGIAR Centers involved in clonal crop conservation to implement global conservation strategies for RTB crops in close collaboration with the Global Crop Diversity Trust (GCDT) and regional and national genebanks.
The Global Musa Genetic Resources Network, in short MusaNet, is a global collaborative framework for Musa genetic resources and a partnership of all key stakeholders, which aims at ensuring the long-term conservation on a cooperative basis.
Conservation methods
Banana (Musa) germplasm is usually seedless and options for its long-term conservation is therefore limited by the vegetative nature of the plant's reproductive system.
- Vegetative methods are the best and more widely used methods of banana conservation.
- Germplasm can be maintained as vegetatively maintained genotypes in fields or screenhouses (field banks), in tissue culture or via cryopreservation (in vitro).
- When the objective is solely to conserve the genes (but not specific combinations of genes), true seeds can be used instead, as long as seeds are produced. Seeds can probably also be conserved with cryo techniques.
- Genes can also be maintained for further use in the form of DNA (DNA banks or lyophilized leaves) or cryopreserved pollen.
Genebanks should have clear priorities on which type of germplasm should be collected, efficiently conserved and securely duplicated.
- Small collections may also give high priority to conserving all material.
- Larger collections should prioritize the conservation of local landraces.
- If core collections have been defined, these must be given the highest priority.
- Conservation of breeding lines and introductions should given lower priority than conservation of local material.
-
The conservation of local landraces must be particularly secured. Possible measures are:
- Use two separate field locations.
- Duplicate in vitro collections in separate locations.
- Maintain a field bank and an in vitro bank.
- Maintain a back up of the in vitro bank under cryopreserved conditions.
- Maintain a back up of the cryopreserved collection in a separate location as black box.
Major banana collections
It is estimated that there are at least 60 major national collections worldwide. Most banana collections manage their accessions in vivo (as full size plants) in the field. A survey carried out by INIBAP in 2006, reported that 25 field collections of banana hold more than 6000 accessions.
About 15 of the surveyed institutes (INIBAP, 2006) host in vitro collections containing slightly more than 2000 accessions, in addition to the Bioversity International Transit Centre (Bioversity ITC) that holds a further 1176 accessions (in vitro).
Cryopreservation methods were first developed at Bioversity ITC in 1996, and work to cryopreserve their entire banana collection started in 2003. The ITC has until now (situation September 2013) cryopreserved 882 accessions belonging to all Musa groups. This represents 63% of the accessions stored in vitro and is thus one of the world’s largest cryopreserved plant collections percentage wise. The National Research Centre for Banana (NRCB) in India and the International Institute of Tropical Agriculture (IITA) in Nigeria are also initiating working on some cryocpreservation methodologies.
Relevant banana collections with rich germplasm diversity or which provide relevant research, expertise, services or capacity building include: BPI, Philippines; CARBAP, Cameroon; CIRAD, France; DHIA, Honduras; DPI&F, Australia; EMBRAPA, Brazil; IEB, Czech Republic; IITA, Nigeria and Uganda; ITC, Belgium; University of Gembloux, Belgium; NARO, Uganda; NRCB, India; PPRI, South Africa; SPC, Fiji.
References and further reading
INIBAP. 2006. Global Conservation Strategy for Musa (Banana and Plantain). Available from: www.croptrust.org/documents/web/Musa-Strategy-FINAL-30Jan07.pdf. Date accessed: 23 March 2010.
MusaNet [online]. Homepage of the MusaNet portal. Available from: https://sites.google.com/a/cgxchange.org/musanet/home Accessed: 25 July 2011.
Panis B, Lambardi M. 2005. Status of cryopreservation technologies in plants (crops and forest trees). International Workshop on "The role of biotechnology for the characterisation and conservation of crop, forestry, animal and fishery genetic resources". Turin, Italy, 5-7 March 2005. pp. 43-54.
Van den Houwe I, Lepoivre P, Swennen R, Frison E, Sharrock S. 2003. The world banana heritage conserved in Belgium for the benefit of small-scale farmers in the Tropics. Plant Genetic Resources Newsletter 135:18-23.
More Articles...
- Characterization of cultivated banana and wild types
- Field bank (regeneration) guidelines for banana
- Health diagnosis and testing of banana genetic resources
- Establishment of aseptic cultures of banana
- Rejuvenation of banana tissue culture
- In vitro conservation of banana genetic resources
- Slow growth storage of banana germplasm
- Cryopreservation of banana apical meristems
- Cryopreservation of banana meristem clusters (cauliflower-like structures)
- Cryo storage for banana genetic resources
Subcategories
-
main
- Article Count:
- 2
-
Registration of Musa
Registration and information systems - Importance and uses
Registration is the first step after acquisition of a sample in any genebank. Collections in genebanks are the genetic base for current and future breeding programs and a source of safety material for distribution to researchers and other users. It is essential that samples are all properly documented from the moment they enter a genebank as well as through all subsequent genebank operations.
A step by step guideline can be seen by clicking here.How should it be done?
Information systems
An information management system must be created in each genebank. This database must be searchable by the genebank curators and staff for specific information through a range of queries.
Numbering and labelling systems
Consecutive alpha numeric or numeric codes must be used for each new accession acquired. This code must be linked to all subsequent information about this sample: passport data, designation status and taxonomic information. The information system must keep a record of genebank operation data, including storage location, stocks, monitoring, health tests and the distribution status. The same system must also manage germplasm orders, shipment related information and files genebanks ‘contacts’ information.
Bar-coding is a useful tool that can compliment a genebank information system.
Extra samples
A separate subset of materials should be kept after registration to be regenerated under greenhouse conditions for harvesting leaf samples that can be processed for DNA/lyophilized leaf bank. These banked leaf materials can serve as a voucher for the germplasm stored in the active and base collections and samples can be made available to users for research in gene discovery and function, marker development and detailed genotypic characterisation. Method to be detailed by INIBAP
Musa Germplasm Information Systems (MGIS)
In 1997, INIBAP laid the basis for a global information system for Musa through the release of MGIS. The aim of the system was to enhance knowledge on Musa diversity, to help rationalizing conservation and to improve the use of banana genetic resources though a facilitated access to comprehensive information.
In 2005, the MGIS database contained key information, including passport data, botanical classification, morpho-taxonomic descriptors and characteristics such as agronomic traits, disease resistance, stress tolerance, biochemical or molecular genetic markers, and plant photographs as well as GIS information on 5188 accessions managed in 18 banana collections (link to the list of collections) around the world making it the most extensive source of information on banana genetic resources.
The database is publicly accessible through the internet at MGIS homepage.htm or at www.mgis.grinfo.net. This global database can be queried on the identity, origin, characteristics and distribution of the individual accessions in the collections. This allows curators of the participant institutions worldwide to share and compare their data. The database is also particularly helpful for various germplasm users namely breeders, researchers and farmer communities, in locating alternative sources of banana germplasm and identifying the most appropriate accessions with particular traits of interest.Homepage of the MGIS website (click on the picture if you wish to go there now)
Further reading:
Van den houwe et al., 2005 (The management of banana (Musa spp.) genetic resources at the IPGRI/INIBAP gene bank: the conservation and documentation status
Calles et al., 2003 (Best Practices for Genebank Management)- Article Count:
- 1
-
Conservation of musa
- Article Count:
- 4
-
In vitro bank for banana
- Article Count:
- 4
-
Cryopreservation for musa
- Article Count:
- 3
-
In the field for musa
- Article Count:
- 3
-
Safety duplication of musa
No. of samples (tubes) per line or cultivar
Size of container
Kind of medium
Amount of medium
Labeling
Placement of label
Bioversity - written directly on cryotubes using pencil
IITA - higher halfLabeling material
Bioversity - not applicable
IITA - marker on parafilm for tubes, tape on polyethylene bagsLabel information
Bioversity - accession ID, freezing date, experiment number
IITA - accession number, line number and date of last introduction
Viability testing
Conditions or timing when the test is conducted
After being one hour in liquid nitrogenNo. of samples for testing
Bioversity - 3 cryotubes per accession (experiment)
IITA – 1-5 seedlingsCriterion for long-term storage
At least 95% certainty that a minimum of 1 plant can be regenerated per experimentTransport
Type of container
Bioversity - dry shipper
IITA – plastic boxesMethod and duration
Bioversity - Air courier or hand carried
IITA - car (4x4) 5 to 6 hours driveConditions
Bioversity - Frozen in liquid nitrogen
IITA - ambientFrequency of shipment
Bioversity - Initially 3 to 4 x a year; once a year thereafter
IITA – 3 to 4 months
Genebank for safety storage
Bioversity - IRD, France & KULeuven, Belgium
IITA - IITA Cotonou, Benin
Storage
Type of storage
Bioversity – Cryopreservation in liquid nitrogen tank
IITA – Tissue culture slow growthType of container
Bioversity - 2mL cryotubes
IITA - Polyethylene bags (13 x 1.3 cm)Temperature (in degrees Celcius)
Bioversity – (-196 oC)
IITA – 18oCLife expectancy of clones
Bioversity - Indefinite
IITA – 4-6 monthsBack-up generator
Bioversity - none
IITA - noneOther features
Availability of liquid nitrogen alarm systemData arrangements
Bioversity - Germplasm ID, inventory of box content sent with samples
IITA - mport permit stating list of accession transferred, endorsed by PQS prior to departure Report at boarder PQS office
Provision for replacement of germplasm
Bioversity - If less than 95% certainty that one minimum plant can be regenerated per experimentProvision for return of germplasm
Bioversity - Loss of samples from LTS at Bioversity ITC; sample provided by the duplication site on Bioversity's requests on a 6 month written notice
IITA - Repatriation permit to ask when needed- Article Count:
- 1









