Viability of forage legume genetic resources
Contributors to this page: ILRI, Ethiopia (Jean Hanson); ICARDA, Syria (Ahmed Amri, Kenneth Street, Ali Shehadeh, Natalya Rukhkyan); GRCTPL, Australia (Richard Snowball); Bioversity International/ILRI, Addis Ababa, Ethiopia (Alexandra Jorge); CIAT, Cali, Colombia (Daniel Debouck).
|
Contents: |
Laboratory method
 Seed germination tests (photo: ILRI) |
Viability test methods are specific for different species. International Seed Testing Association rules should be used when available. Specific information is available for many species (see Species Compendium).
Type of test
- Standard germination tests (see list for species specific information).
or
- Sequential germination tests (these are less accurate, but require less seeds and are suitable in case of limited number of seeds). Use at least 40 seeds per test. If less than 30 seeds germinate, programme the accession for regeneration.
Number of seeds and replicates
|
The text for these flipbooks was extracted from: Rao NK, Hanson J, Dulloo ME, Ghosh K, Nowel D and Larinde M. 2006. Manual of seed handling in genebanks. Handbooks for Genebanks No. 8. Bioversity International, Rome, Italy. 147pp.
|
International Seed Testing Association rules should be used when available.
- ISTA recommends four replications of 100 seeds.
- A minimum number of four replications of 50 seeds is acceptable, when seeds are limited.
- The number of seeds should be practical (depending on the seeds available) and enough to provide statistically significant results.
- Wild species often have limited numbers of seeds to use for testing.
- For the sequential test use 40 seeds.
Pre-treatment
International Seed Testing Association rules should be used when available.
- Viability test methods are specific for different species (see list for specific information).
Media
International Seed Testing Association rules should be used when available.
- Viability test methods are specific for different species (see list for specific information).
Temperature
International Seed Testing Association rules should be used when available.
- Viability test methods are specific for different species (see list for specific information).
Light
International Seed Testing Association rules should be used when available.
- Viability test methods are specific for different species.
- A 12 hour light period should be used to simulate tropical day length conditions.
Duration of test
International Seed Testing Association rules should be used when available.
- Viability test methods are specific for different species (see list for specific information).
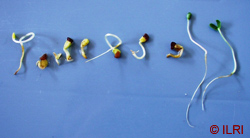
The picture above shows abnormal (left) and normal (the last two on the right) legume seedlings. (photo: ILRI) |
Others
- Germination is complete when the seedling can be judged as normal (ISTA, 2006), i.e. having a developed shoot and root (see image below).
Monitoring intervals
Legume seeds are usually long-lived. The sampling interval should be spaced so as not to allow seeds to fall below 65% viability but not so frequent as to waste seeds for unnecessary testing:
- Every ten years above 80% germination.
- Every five years below 80% germination or when longevity is not known.
- Seeds should be regenerated when viability reaches 65%.
Recording information during viability testing
The following information must be recorded for each testing step:
- Accession number (ID number).
- Lot number (ID number).
- Taxonomic identification.
- Date of test (the date that the test was commenced).
- Number of replications (the number of replicates in the test).
- Number of seeds per replication (the number of seeds in each replicate of the test).
- Pre-treatment (pre-treatments used for the test. More than one can be used in conjunction).
- Media (the media for the test).
- Temperature (the temperature used during the test in degrees centigrade. If an alternate temperature is used this temperature usually refers to the temperature during the day period, unless stated otherwise).
- Alternate temperature (if more than one temperature is used, the temperature in degrees centigrade of the alternate period. The alternate temperature usually corresponds to the dark period).
- Length of dark (the number of hours that there is no light. This usually corresponds to the period at lower temperature, when two temperatures are used).
- Length of test (the number of days during which the test was continued).
- Percentage germination (the average percentage germination over all replicates, calculated from the number germinated per replicate).
- Tolerance (a measure of the statistical accuracy of the test according to the standard tolerance tables).
- Percentage dormancy (the average percentage of hard seeds which are not rotten, over all replicates).
- Test reference (a consecutive number given each time that seed lot is tested over time).
Describes the recommended monitoring methods to assure minimum viability and quantity of seeds in storage.
- Do routine tests for seed viability at regular intervals (monitoring methods for viability should minimize use of seeds while providing a good indication of reduction of viability).
- The stock control system should be queried to determine accessions for regeneration.
-
Accessions can be flagged (marking accessions in the database helps to avoid distribution of accessions by mistake and quickly identify accessions for regeneration) in the database as:
- (A) Accession is available for distribution.
- (L) Low stock; the accession is available for distribution but regeneration should be planned.
- (S) Low number of seeds; no distribution is allowed; the sample should be regenerated.
- Genebank facilities should be checked for good working order or damage and preventive maintenance should be done on equipment (regular monitoring of equipment and buildings allows early identification of problems and preventive maintenance before failure).
Monitoring frequency
- This is set according to the minimum quantity and minimum viability of seeds below which they need to be regenerated.
Critical quantity
- A minimum of 600 seeds (this quantity allows two regeneration attempts using 300 seeds giving a high probability to have 100 plants to maintain genetic integrity of accessions, even for seeds with 50% viability).
- Where possible it is better to regenerate when seed quantities reach 1000 to mitigate risks.
Critical germination level
- 65% germination percentage for wild species (recommended by FAO/IPGRI, 1994).
Recording information during routine monitoring
The following information should be recorded for each step:
- Accession number (ID number).
- Lot number (ID number).
- Weight of seeds (weight of seeds on hand).
- 1000 seed weight (weight of one thousand seeds to be used for calculating number of seeds from weights).
- Percentage of germination (the average percentage germination over all replicates, calculated from the number germinated per replicate).
- Stock control flag (code to indicate the stock level and distribution).
- Availablity of material flagged (code to indicate the stock level – see above)
- Regeneration flag (code to indicate if regeneration required).
References and further reading
Akinola JO, Afolayan RA, Olorunju SAS. 1991. Effects of storage, testa colour and scarification method on seed germination of Desmodium velutinum (Willd.) DC. Seed Science and Technology 19:159-166.
FAO/IPGRI. 1994. Genebank standards. Food and Agriculture Organization of the United Nations, Rome and International Plant Genetic Resources Institute, Rome. Available in English, Spanish, French and Arabic.
International Seed Testing Association [homepage of (ISTA)] [online]. Available from: http://www.seedtest.org/en/home.html. Date accessed: 17 Jan 2011.
ISTA. 2006. Handbook on Seedling Evaluation, 3rd Edition.
Souza FHD, Marcos-Filho J. 1993. Physiological characteristics associated with water imbibition by Calopogonium mucunoides Desv. seeds. Seed Science and Technology 21:561-572.
Thormann I, Metz T, Engels JMM. 2004. The Species Compendium (release 1.0; December 2004). [online] Available from: http://www.bioversityinternational.org/databases/species_compendium_database/index.html. Date accessed: 09 April 2013.
Wang YR, Hanson J. 2008. An improved method for breaking dormancy in seeds of Sesbania seban. Cambridge University Press. Experimental Agriculture. Vol. 44, pp. 185–195. Abstract available from: http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=1842236. Date accessed: 17 Jan 2011.
Wang YR, Hanson J. 2008. The impact of temperature on seed germination in diverse accessions of 4 wild Vigna species. Proceedings of the XXI International Grasslands Congress, Multifunctional Grasslands in a Changing World. Hohhot, China, June 2008, p. 557.
Wang YR, Hanson J, Wolde YM. 2007. Effect of sulfuric acid pretreatment on breaking hard seed dormancy in diverse accessions of five wild Vigna species. Seed Science and Technology 35:550-559.
Comments
- No comments found





Leave your comments
Post comment as a guest