CGKB News and events Banana
|
Importance and origin Banana (Musa spp.) is one of the most ancient fruit crops known and used by man. It originated in South-East Asia and was first domesticated some 7000 years ago. Today it is grown in every humid tropical and many sub-tropical regions. It is the fourth most valuable food crop after rice, wheat and maize. The fruit crop provides a staple food source for 400 million people, most important in East–Africa. About 95 million metric tons of bananas are harvested annually around the world, 30% of these being plantains. About 90% of the total production takes place on small-scale farms and it is used for home consumption or in domestic markets. The remaining 10% (dessert bananas) are mostly produced in Latin-America and Caribbean and commercialized in world trade.
Production of banana in the world
|
|
|
How is it consumed? For many people in the tropics, bananas are an essential component in their daily diet.
Bananas are essential for many people in the tropics
|
|
|
Which types exist? Bananas evolved from inter-and intraspecific hybridization between two diploid wild species of the genus Musa, sp. acuminata (AA) and sp. balbisiana (BB), the only species that set seeds. These crosses produced edible cultivars (all female sterile with various levels of male sterility) currently cultivated, with the following genomic configuration:
|
|
|
Further reading: Simmonds and Shepherd, 1955 |
Rejuvenation of banana tissue culture
Contributors to this page: Bioversity International, Belgium (Ines Van den Houwe); IITA, Nigeria (Dominique Dumet, Badara Gueye).
|
Greenhouse regeneration |
Why regeneration/rejuvenation of stored tissue cultures
Regeneration and rejuvenation of an accession involves the transfer of the accessions to the greenhouse and field in order to check their trueness-to-type (identity and conformity). Rejuvenation is the replacement of the tissue cultures in storage by a set of quality material derived from the verified material. Often both activities are combined.
Using field established plants for harvesting shoot tips to rejuvenate the accession in storage would require re-indexation for viruses, as the plants could have been exposed to viruses.
In order to rejuvenate an accession without having to go through re-indexation of the material, in vitro plants from the accession should first be established in the greenhouse.
It is recommended that this standard procedure involving regeneration and rejuvenation of accessions should be carried out for any accession being maintained continuously in vitro for more than ten subculture cycles (or ten years in MTS):
Regeneration of plants in the greenhouse
- From the set of cultures in storage, a representative sample of five cultures should be randomly selected and regenerated into five rooted plantlets. These plants should be planted out in the greenhouse.
Decapitation of suckers
- Approximately nine months after the planting date, when the sucker diameter is at least 4.5 cm, greenhouse plants should be decapitated in order to stimulate the outgrowth of new shoots.
- The corms should be taken out of the pots and the adhering soil must be gently removed, without damaging the sleeping buds on the corm.
- The roots should be cut back to about 5 cm from the corm surface, leaving the corm tissue and dormant buds intact.
- Leaf sheaths should be cut back to about 5cm above the corm leaving all lateral buds covered.
- The apical shoot tip should be removed by excising a cube of tissue of 2x2x6 cm³ from the upper centre of the corm.
- The disinfection, shoot tip culture initiation and bacterial indexing procedures that must be applied next are described in sample procedures.
- In order to avoid fungal deterioration of the prepared corms, the basal part must be sprayed with a fungicide and the upper part must be protected at the cut surfaces with a thin layer of a Vaseline-fungicide mixture.
- Corms should be ‘replanted’ in five litre pots filled with a mixture of peat and soil, and topped up with a 10 cm layer of vermiculite.
- The decapitated corms should be kept in a greenhouse at an ambient temperature ranging between 18°C (night) and 26°C (day) and a RH of 70-90%.
- Four months after decapitation, one single sucker per accession, producing the highest number of new and normal looking shoots must be selected.
- Shoots of at least one centimetre are suitable for shoot tip isolation and tissue culture initiation.
- All shoot tips should be harvested and re-initiated in vitro following the protocols described for sample procedures.
In vitro plants derived from two shoot tips must be sent to the field for verification. If these plants are confirmed true-to-type (after two growing cycles), the remaining shoot-tips from the same sucker can be further multiplied in vitro and replace the old set of replicates in cold storage.
The whole regeneration/rejuvenation cycle takes about 20-22 months.
Field verification and characterization of accessions
- Accessions must be verified for their trueness-to-type in the field. Ideally, they are grown in a field collection next to the original mother plant and observed for their morphological characteristics (using a minimal set of descriptors) during two subsequent growth cycles.
- Accessions that are confirmed to possess the same characteristics as the original genotype can be declared true-to-type and they are renewed in MTS following the rejuvenation process described above.
- Accessions identified as off-types with no value, or accessions which are found to be mislabelled (ML), must be discarded and replaced with the original true-to-type material from the donor source.
References and further reading
Van den Houwe I, Panis B, Arnaud E, Markham R, Swennen R. 2006. The management of banana (Musa spp.) genetic resources at the IPGRI/INIBAP gene bank: The conservation and documentation status. In: Segers H, Desmet P, Baus E, editors. Tropical Biodiversity: Science, Data, Conservation. Proceedings of the 3rd GBIF Science Symposium. Brussels, Belgium, 18-19 April 2005. pp.141-150.
In vitro conservation of banana genetic resources
Contributors to this page: Bioversity International, Belgium (Ines Van den Houwe); IITA, Nigeria (Dominique Dumet, Badara Gueye).
What is in vitro conservation
During the last 40-50 years in vitro techniques have been increasingly used for plant propagation. They consist in growing and multiplying parts of plants in flasks or tubes in artificial media, under controlled environments and sterile conditions.
Common banana in vitro techniques used in conservation are listed below:
- Slow growth storage – using tissue culture techniques and growth retardant conditions (temperature, light, chemicals).
- Cryopreservation.
Where is it used
An increasing number of countries has invested in tissue culture facilities for the propagation of clonal crops, including banana.
Initially, traditional tissue culture techniques (shoot tip and meristem culture) were used as a tool for the elimination of pests and diseases, rapid plant propagation and for the exchange of clean germplasm. As institutes became more experienced with these techniques, and the number of accessions in collections steadily increased, the available techniques were optimized and extensively adopted for slow growth conservation of germplasm. Until now however very few genebanks have initiated banana conservation activities using cryopreservation techniques.
When should it be used
In vitro conservation techniques should be used whenever technical expertise and facilities are available. They are generally more economic and less risky in a long-term perspective.
- To conserve plant parts of banana germplasm that can mostly be propagated vegetatively
- As a viable alternative to complement and reduce the large space required for field banks.
- In vitro conservation has low space requirements and minimal possibility of losses due to edaphic factors.
- To duplicate material contained in field banks.
- To replace field banks.
- To allow international germplasm exchange.
- To ensure a more secure conservation of germplasm for future generations.
- Costs (qualified labour, energy, supplies and infrastructure) are highly dependent on location and ecomomies of scale should be considered when taking in vitro conservation into consideration.
How should it be done
- It requires specialized laboratories and equipment with very skilled technicians and researchers.
- It also requires adaptive technologies for some more reluctant species.
- It requires sterile conditions and very well controlled artificial growth environments.
- It requires high initial investments but relatively low maintenance costs in a long-term perspective.
- In vitro conservation should only be considered if the laboratory forms part of a conservation strategy involving also other crops.
References and further reading
Benson E, Harding K, Debouck D, Dumet D, Escobar R, Mafla G, Panis B, Panta A, Tay D, Van denhouwe I, Roux N 2011. Refinement and standardization of storage procedures for clonal crops - Global Public Goods Phase 2: Part III. Multi-crop guidelines for developing in vitro conservation best practices for clonal crops. Rome, Italy: System-wide Genetic Resources Programme. Available here.
Benson E, Harding K, Debouck D, Dumet D, Escobar R, Mafla G, Panis B, Panta A, Tay D, Van denhouwe I, Roux N 2011. Refinement and standardization of storage procedures for clonal crops - Global Public Goods Phase 2: Part II. Status of in vitro conservation technologies for: Andean root and tuber crops, cassava, Musa, potato, sweetpotato and yam. Rome, Italy: System-wide Genetic Resources Programme. Available here.
Benson E, Harding K, Debouck D, Dumet D, Escobar R, Mafla G, Panis B, Panta A, Tay D, Van denhouwe I, Roux N 2011. Refinement and standardization of storage procedures for clonal crops - Global Public Goods Phase 2: Part I. Project landscape and general status of clonal crop in vitro conservation technologies. System-wide Genetic Resources Programme. Available here.
Calles T, Dulloo ME, Engels JMM, Van den Houwe I. 2003. Best Practices for Germplasm Management - A new approach for achieving genebank standards. Technical Report. International Plant Genetic Resources Institute, Global Crop Diversity Trust, Rome, Italy. Available here.
Mafla G. 1994. Conservación de germoplasma In vitro. In: King C, Osorio J, Salazar L, editors. Memorias I Seminario Nacional sobre Biotecnología. Universidad del Tolima. Colombia. pp. 65-77.
Roca WM, Chaves R, Marin ML, Arias DI, Mafla G, Reyes R. 1989. In vitro methods of germplasm conservation. Genome 31 (2):813-817.
Roca WM, Mafla G, Segovia RJ. 1991. Costo mínimo de un laboratorio de cultivo de tejidos vegetales. In: Roca WM, Mroginski LA, editors. Cultivo de tejidos en la agricultura: Fundamentos y Aplicaciones. pp. 912-920.
Szabados L, Nuñez LM, Tello LM, Mafla G, Roa JC, Roca WM. 1991. Agentes gelanitizadores en el cultivo de tejidos. In: Roca WM, Mroginski LA, editors. Cultivo de tejidos en la agricultura: Fundamentos y Aplicaciones. pp. 79-93.
Slow growth storage of banana germplasm
Contributors to this page: Bioversity International, Belgium (Ines Van den Houwe); IITA, Nigeria (Dominique Dumet, Badara Gueye).
|
Contents: |
Sample processing for tissue culture banks
Multiplication of propagules for conservation
When an accession has successfully passed the initiation phase (click here for details), then it is ready to be multiplied for storage, either for normal growth or slow growth conservation. This multiplication phase is also required for rapid propagation of selected materials for research or distribution.
Starting material
- The desired number of proliferating cultures must be obtained through repeated subculturing of propagules on proliferation medium.
- For rapid multiplication purposes, shoot tip cultures should be subcultured at 4-6 week intervals.
- Clusters of multiple shoots should be divided into individual or smaller groups of 2-3 micro-shoots and/or buds.
- Superfluous corm tissue and blackened tissue should be trimmed.
- The shoots must be shortened to a size of 5-7 mm in height.
- Each excised shoot tip or group of shoots /buds should be transferred onto a fresh pre-sterilized multiplication medium.
- The cultures should be incubated at an ambient temperature of 28±2°C and a light intensity of 64 µmol m-2 s-1.
- After 2-4 weeks, new lateral shoots and/or buds start to develop.
- After a further two weeks, subculturing can be repeated.
This propagation phase of an accession may vary from a few weeks to a few months as the multiplication rate strongly depends on the genomic group to which the accession belongs and is influenced by the composition of the medium (particularly the cytokinin concentration), the explant size, age of culture and the size of the culture vial.
Visual inspection of plant materials
- As the storage capacity strongly depends on the initial quality of the cultures, the general performance of each culture should be visually assessed: vigour, absence of fungal and bacterial contamination, chlorosis, blackening, necrosis of the tissue before transfer to slow growth storage.
Disposal of contaminated materials
- Contaminated and low quality cultures should be immediately discarded. If the entire set is below standard because at least one of the criteria is not met, the cultures should be re-propagated onto a new medium.
- If sufficient suitable cultures are available for storage, the culture tubes should be sealed with a few layers of parafilm and transferred to slow growth conditions.
Watch the video illustrating the aseptic subculturing process of banana shoots
Or view the video on Youtube
Storage for tissue culture banks
- When the desired number of cultures for slow growth storage is obtained, the set of replicate cultures must be carefully observed after 2-3 weeks of growth.
Sample specifications
- An optimal sample size should be determined based on the purpose of the collection, taking in account most risks of possible losses.
- Based on statistical data, the proper sample size was determined between 12 and 24.
- An optimal number of 16-20 replicate shoot cultures per accession should be kept in storage.
- An accession tray containing an estimated reasonable number of replicate cultures should be placed in its assigned slow growth storage location within the storage growth room.
Container specifications
- Individual cultures should be stored in glass test tubes (150 mm height and 2.5 mm diameter), closed with a plastic kaput and sealed with several layers of parafilm in order to limit evaporation.
Growth media
- The test tubes should contain 20 ml of proliferation inducing culture medium composed of the MS Murashige and Skoog -mineral salts and vitamin mixture (Murashige and Skoog 1962) and be supplemented with 30 g/l sucrose; two growth regulators should be incorporated into the medium: cytokine in relatively high concentration (2.25 mg/l BAP) in order to induce multiple shoot/bud formation and an auxin (0.175 mg/l IAA) and solidified with 2 g/l Gelrite. (Van den houwe et al. 1995).
- The medium should be adjusted to pH 6.2 prior to autoclaving the medium.
Culture facility regimes
- The accession tissue cultures should be stored at an ambient temperature of 16°±1°C and at reduced light intensity of 25 µmol m² s-1.
- A 24-hour light regime should be applied and the relative humidity of the storage room should be kept at 75%.
Storage duration
If the above physical and chemical storage conditions are followed, an average period of 12 months can be expected before re-culturing is required. These storage conditions are minimal growth conditions that proved to be acceptable for most genotypes. Not all accessions and genotypes, however, respond equally well to the applied conditions. (Further reading: Van den houwe et al. 1995).
System for tracking materials/inventory system during tissue culture storage
- The cultures should be inventoried every time subculturing is carried out.
Monitoring the performance of tissue cultures in slow growth storage
Need for subculturing multiplication of stored cultures
Quantitative and qualitative criteria should be considered to define the moment that an accession should be recycled after the material has been maintained for a given time in storage.
- When the number of cultures maintained is reduced below the threshold value of 12, the accession should be moved to the transfer room for subculturing.
- If the number of cultures is higher than 12 but all cultures are characterized by an advanced stage of deterioration, necrosis, chlorosis, blackening or hyperhydrity, the accession should also be moved to the transfer room for subculturing (see routine monitoring methods).
- Ideally, one accession should be re-propagated into a set of 20 fresh shoot culture replicates, performing one subculture cycle. If, however, the number of newly established cultures is lower than 16, subculturing should be repeated to increase their numbers.
- In order to minimize the risk of selecting variant plant material from the remaining set of cultures, at least one shoot tip (with a maximum of three) should be isolated from each individual viable and healthy culture.
- Each individual shoot tip should be transferred to another recipient containing fresh culture medium.
- For safety reasons it is always recommended to hold 2-4 viable and healthy cultures of the previous subculture cycle as spare materials until it is known that the newly subcultured set is healthy and growing.
- Propagules should initially grow for 2-3 weeks under normal growth conditions at an ambient temperature of 28°C and under a PPF of 63 µmol m-² s-1 (with a 24-hour photoperiod).
The spare cultures from the previous storage cycle can be discarded when the new set of cultures are transferred to the cold storage conditions described above.
Inventory and monitoring of stored cultures
Accessions growing in tissue culture need to be inventoried and monitored on a timely basis in order to assess the number of healthy cultures remaining in storage and to determine the need for subculturing. The interval for routine visual monitoring of an active Musa collection should be about one month. Each individual culture in the accession tray should be checked visually and unsuitable cultures removed.
Checking the quality of the plantlets
The following factors should be taken into account to determine the quality of stored accessions:
- Viability.
- Vigour.
- Necrosis (of the leaves and apex).
- Chlorosis.
- Blackening of tissue / medium discoloration.
- Hyperhydricity.
- Ethiolation.
- Contamination
Plantlets of unacceptable quality should be immediately discarded.
Checking the number of replicates
- When the number of remaining healthy cultures falls below 12 or if the cultures are of unacceptable quality, the accession should be re-cultured under normal growth conditions in the following month.
- If a critical level of four or less cultures is reached, the accession must be considered at risk and immediate action should be taken to re-propagate or rescue the accession.
- In the case of contamination of all replicates, material should be transferred to the greenhouse, if plantlets are available. Otherwise, the propagules are regenerated and/or subjected to a decontamination treatment.
- If all remaining propagules are of poor quality, the material should either be immediately subcultured or rescued by being transfered into the greenhouse.
- If more than 12 cultures of the desired quality are left over in storage, the conservation cycle should continue for another month.
Monitoring genetic stability
Unless germplasm is regularly regenerated and transferred to the field for morphological observations, combined with the use of cytological techniques, genetic stability of a certain sample cannot be ascertained. Occasionally, abnormalities can be assessed in the in vitro samples.
In vitro assessment of variation
One of the criteria for efficient in vitro storage of germplasm is the maintenance of original genotypes over long periods of time. Although organized cultures (meristems, shoot and root-tip cultures) are believed to be genetically more stable than disorganized cultures (cell suspensions, protoplasts, callus, differentiated cells) variation appears to be relatively widespread in micro-propagated plants.
Factors like the culture mode, time in culture, number of subculture cycles, genotype and composition of the culture medium are known to influence the occurrence of somaclonal variation. The type and frequency of variation in micropropagated banana plants is known to be genotype and cultivar dependent.
- Monthly routine monitoring of the stored tissue culture samples should be carried out, making visual examinations for growth abnormalities.
- Some types of somaclonal variation (different degrees of dwarfism) can be observed at the tissue culture level.
- However, most types (leaf variegation, stem discoloration and particularly mutations expressed at the inflorescence and fruiting level - prolonged juvenility, small bunch, shortened fingers) cannot be assessed in vitro and require the regeneration of plants under field conditions. (Van den Houwe and Panis 2000).
Observations under greenhouse and field conditions (regeneration)
- When the accession has been continuously stored in vitro for over ten years, or when the accession is kept for more then ten subculture cycles in slow growth storage, the accession should be regenerated and observed morphologically for trueness-to-type under greenhouse and field conditions during at least two growing cycles.
- The morphological and taxonomic characteristics of the plants must be compared with those of the original accession.
References and further reading
Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco cell cultures. Physiologia Plantarum 15:473–497. Available for purchase here.
Strosse H, Van den houwe I, Panis B. 2004. Banana cell and tissue culture - review. Mohan Jain S, Swennen R (ed). Banana Improvement:Cellular, Molecular Biology, and Induced Mutations. Science Publishers Inc., Enfield, NH, USA:1-12. www.fao.org/docrep/007/ae216e/ae216e03.htm#bm03.1
Van den Houwe I, De Smet K, Tezenas du Montcel H, Swennen R. 1995. Variability in storage potential of banana shoot cultures under medium term storage conditions. Plant Cell, Tissue and Organ Culture 42:267-274. An abstract and full preview of the publication is available here.
Van den Houwe I, Panis B. 2000. In vitro conservation of banana: medium term storage and prospects for cryopreservation. Razdan MK, Cocking E, editors. Conservation of Plant Genetic Resources in vitro. Vol. 2. M/S Science Publishers, U.S.A. pp. 225-257.
Cryopreservation of banana apical meristems
.jpg) View the full publication on Cryopreservation of Musa germplasm by clicking on the icon above (1.35 MB) |
Contributors to this page: Bioversity International, Belgium (Bart Panis).
The content of this page was extracted from Panis B. 2009. Cryopreservation of Musa germplasm: 2nd edition. Technical guidelines No. 9 (F. Engelmann and E. Benson, eds). Bioversity International, Montpellier, France.
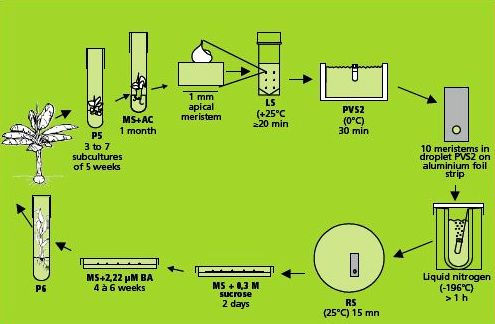
Cryopreservation of in vitro grown shoot tips of banana through vitrification was originally reported by Thinh and co-workers (Thinh et al. 1999). Using this method, regeneration percentages were often low and unpredictable. Therefore, the technique was further improved and adapted at KULeuven to make it applicable to a wide variety of cultivars (Panis et al. 2005).
This method is illustrated in the figure below.
|
Cryopreservation of individual meristems (photo: KULeuven/Bioversity) |
Preparation of plant materials
|
Production of robust, rooted in vitro plants of banana cv. ‘Williams’ (photo: KULeuven/Bioversity) |
Preparation of in vitro plantlets
All accessions were obtained from the Bioversity Musa germplasm in vitro collection (KULeuven, Belgium). This collection contains edible banana cultivars, as well as wild relatives.
- Shoot cultures are grown in 25x150 ml test tubes on 25 ml p5 medium. P5 medium contains semi-solid Murashige and Skoog (MS) medium supplemented with 30 g/L sucrose, 10 μM BA and 1 μM IAA and solidified with 2 g/L gelrite (Banerjee and de Langhe 1985). They are cultured at 25±2°C under continuous 50 μE m-2 s-1 illumination provided by 36W Osram cool-white fluorescent tubes. The pH is adjusted to 5.8 prior to autoclaving.
- From these multiplying cultures, shoots of 3-5 cm in length are separated and transferred to rooting medium in test tubes. The rooting medium has the same composition as p5, but it is devoid of plant growth regulators and supplemented with 0.5 g/L active charcoal.
- After one month, robust and well rooted in vitro plants are obtained with a corm diameter of 5-8 mm that forms an appropriate source for the excision of apical meristems (see photo).
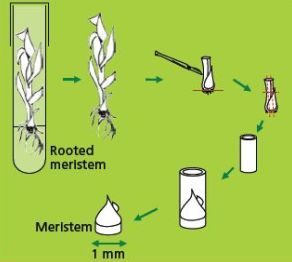
Dissection and selection of apical meristems
Like many other monocots, banana apical meristems are tightly covered with several layers of whitish, tubular, immature leaves.
- Individual apical meristems are excised under a binocular microscope. Leaves are removed one by one until the apical dome is visible, but still partially covered by 1-2 young leaf primordia (see figure and photo below).
- The leaf base (corm tissue) is 1 mm in diameter. In order to excise corm pieces of exactly this size, a millimetre grid graph paper is placed underneath the transparent sterile plastic Petri dish in which the meristems are excised.
- The dissected meristems are transferred into the loading solution (in the dark at room temperature). Tips that are slightly damaged or are not in the correct stage (i.e. the meristem is too much or too little covered by leaf primordia) are excluded from cryopreservation.
A skillful and trained technician can isolate a maximum of ten meristems an hour (roughly, six minutes/isolated meristem).
|
Illustration of meristem isolation. Leaves are removed one by one until the apical dome is visible but still partially covered by 1-2 young leaf primordia |
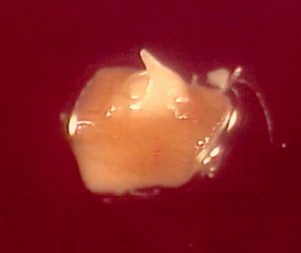
Partly-covered apical meristems of banana cv. ‘Williams’ (photo: KULeuven/Bioversity)
|
Cryopreservation through droplet vitrification
Loading, dehydration and rapid freezing
|
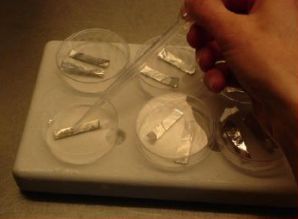
Transfer of meristems to a droplet of PVS2 solution (of about 15 μl) on a strip of aluminium foil (5x20 mm) with a 2 ml plastic Pasteur pipette (photo: KULeuven/Bioversity) |
- The filter sterilised loading solution contains 2 M glycerol and 0.4 M (= 136.8 g/L) sucrose dissolved in MS medium (pH 5.8). The excised meristems are left in the loading solution in a 20 ml plastic vessel until all of them are dissected. The exposure time thus varies between 20 min and 5 hrs. Previous research has shown that regrowth of banana meristems is not influenced by loading solution exposure time (Panis et al. 2005). Although the precise mechanism of loading is not yet fully understood, it has been proven for different plant species that loading can dramatically enhance the tolerance of isolated meristems to dehydration by the vitrification solution (Matsumoto et al. 1994, Takagi et al. 1997).
- After loading, the solution is replaced by ice-cooled PVS2 solution. The PVS2 solution consists of 30% (w/v) (3.26 M) glycerol, 15% (w/v) (2.42 M) ethylene glycol (EG), 15% (w/v) (1.9 M) DMSO and 0.4 M (= 136.8 g/L) sucrose (Sakai et al. 1990).
- All these compounds are dissolved in MS medium, pH adjusted to 5.8, followed by filter sterilization. The meristems are subjected to the PVS2 solution for 30-40 min. at 0°C.
- Five min. before the end of the treatment, ten meristems are transferred individually to one droplet of PVS2 solution (of about 15 μl) on a strip of aluminium foil (5x20 mm) with a 2 ml plastic Pasteur pipette (see photo).
- To keep the temperature of the strip around 0°C during the manipulations, the aluminium strip is placed in a plastic Petri dish placed on top of a frozen cooling element. After the PVS2 treatment, the aluminium strip is plunged into liquid nitrogen with a fine forceps.
- For permanent cryostorage, the frozen foil is quickly transferred to a 2 ml cryotube filled with liquid nitrogen and closed.
Storage, re-warming and unloading
- The meristems are kept in liquid nitrogen for at least 20 min. For re-warming, aluminium foil strips are rinsed in 10 ml unloading solution in a small Petri dish at room temperature. After a few seconds, meristems are released from the strip of aluminium foil and kept for another 15 min in the unloading solution. As such, the toxic PVS2 solution is removed whilst re-warming and replaced by a less-toxic unloading solution. The unloading solution consists of 1.2 M (= 410.4 g/L) sucrose dissolved in MS medium (pH 5.8).
Recovery
- Unloaded meristems are placed onto two sterile filter papers on top of semi-solid hormone-free MS medium containing 0.3 M (=102.6 g/L) sucrose.
- After two days, the meristems are transferred onto regeneration medium without filter papers. The first week of culture always takes place in the dark.
Four to six weeks following cryopreservation, four types of reactions can be distinguished:
(i) white shoot tips resulting from an immediate death of the tissue without blackening.
(ii) completely or partially black shoot tips, indicating that there was enzymatic reaction following cryopreservation (production and oxidation of polyphenols).
(iii) unorganized callus growth, representing the outgrowth of small isolated areas of the apical dome and/or primordial tissues.
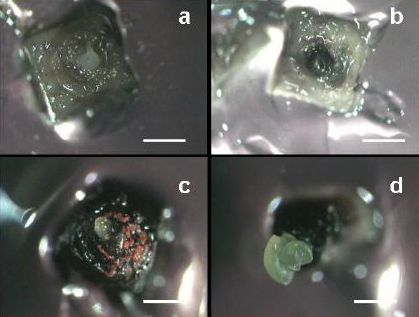
(iv) shoot tip regeneration resulting from the survival of a substantial part of the apical dome (see figures a-d).
 |
|
|
Reaction of apical meristems towards cryopreservation 30 days after re-warming (a) No growth; the meristem remains white; (b) Blackening without further growth; apical dome reacts by formation of polyphenolic compounds that oxidize; (c) Callus formation; watery, non-morphogenic callus; and (d) Shoot regeneration (bar = 600 μm)(photo: KULeuven/Bioversity) |
One month after re-warming, a 0.5 cm long shoot can be observed (see photo). Calluses never produced shoots.
|
Recovered shoots from cryopreserved apical meristems of banana cv. ’Williams’ 1 month after re-warming |
Discussion and perspectives
Different Musa genotypes have been cryopreserved using this protocol (Thinh et al. 1999, Panis et al. 2005). Post re-warming regeneration percentages range between 20% and 85% (with an average of 53%). These recovery percentages are relatively genotype independent. It was, however, observed that genotypes with more B-genome survive significantly better than those with solely the A-genome (Panis et al. 2005). Microscopic observation of the recovery of cryopreserved meristems (Helliot et al. 2003) has revealed that:
(i) the whole dome of the isolated meristem withstands exposure to liquid nitrogen.
(ii) no regenerable post cryopreservation callus is formed. Therefore, it would appear that somaclonal variation is unlikely to occur.
The main limitations to this procedure are as follows:
- Post cryopreservation viability/regeneration percentages vary depending on the operator. Considerable experience in the dissection of tiny and fragile banana apical meristems is required before this cryopreservation protocol can be applied (using this method only 60 meristems can be excised and cryopreserved in one day).
- Low regrowth may be due to low quality meristems (tips that are slightly damaged or are not at the correct stage or too much or too little covered by leaf primordia). The quality of the donor plants (more light, fewer plantlets in containers) should be carefully monitored.
- Using the droplet vitrification method, some researchers have shown some concern with respect to the effects of the direct contact of liquid nitrogen with the plant material. Supposed problems like contamination and loss of material were, however, never encountered.
This droplet vitrification protocol was recently successfully applied to a wide range of plant species such as potato, ulluco, sweet potato, chicory, strawberry, taro, Pelargonium L., date palm, thyme, olive, Byrsonima, snowdrop, apple and hop and might thus be considered as the first generally applicable protocol (Panta et al. 2006, Gallard et al. 2008, Sant et al. 2008, Feki et al. 2011, Sánchez-Romero and Panis 2009, Marco-Medina et al. 2010, Condello et al. 2011, Maslanka et al. 2013, Coutinho Silva et al. 2013).
References and further reading
Banerjee N, De Langhe E. 1985. A tissue culture technique for rapid clonal propagation and storage under minimal growth conditions of Musa (banana and plantain). Plant Cell Reports 4:351-154.
Condello E, Caboni E, André E, Piette B, Druart P, Swennen R, Panis B. 2011. Cryopreservation of apple in vitro axillary buds using droplet-vitrification. CryoLetters 32:175-185.
Coutinho Silva L, Paiva R, Swennen R, André E, Panis B. 2013. Shoot-tips cryopreservation by droplet vitrification of Byrsonima intermedia A. juss.: a wooden tropical and medicinal plant species from Brazilian Cerrado. CryoLetters 34: 338-348.
Feki L, Bouaziz N, Sahnoun N, Swennen R, Drira N, Panis B. 2011. Palm cryobanking. CryoLetters 32:451-462.
Gallard A, Panis B, Dorion N, Swennen R, Grapin A. 2008. Cryopreservation of Pelargnonium apices by droplet-vitrifications. CryoLetters 29 (3):243-251.
Helliot B, Swennen R, PouMay Y, Frison E, Lepoivre P, Panis B. 2003. Ultrastructural changes associated with cryopreservation of banana (Musa spp.) highly proliferating meristems. Plant Cell Reports 21:690-698.
Marco-Medina A, Casas JL, Swennen R, Panis B. 2010. Cryopreservation of Thymus moroderi by droplet vitrification. CryoLetters.
31 (1):14-23.
Maslanka M, Panis B, Bach A. 2013. Cryopreservation of Galanthus elwesii Hook. apical meristems by droplet vitrification. Cryo Letters, 34: 1-9.
Matsumoto T, Sakai A, Yamada K. 1994. Cryopreservation of in vitro-grown apical meristems of wasabi (Wasabi japonica) by vitrification and subsequent high plant regeneration. Plant Cell Reports 13:442-446.
Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco cell cultures. Physiologia Plantarum 15:473–497.
Panis B. 2009. Cryopreservation of Musa germplasm: 2nd edition. Technical Guidelines No. 9 (F. Engelmann and E. Benson, eds). Bioversity International, Montpellier, France. Available here.
Panis B, Piette B, Swennen R. 2005. Droplet vitrification of apical meristems: a cryopreservation protocol applicable to all Musaceae. Plant Science 168:45-55.
Panta A, Panis B, Ynouye C, Criel B, Swennen R, Roca W. 2006. Improvement of potato cryopreservation for the long-term conservation of Andean landraces at CIP. 43rd Meeting of the Society for Cryobiology in association with the Society for Low Temperature Biology. Hamburg, Germany, 24-27 July 2006.
Sakai A, Kobayashi S, Oiyama I. 1990. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Reports 9:30-33.
Sánchez-Romero C, Swennen R, Panis B. 2009. Cryopreservation of olive embryogenic cultures. CryoLetters 30 (5):359-372.
Sant R, Panis B, Taylor M, Tyagi A. 2008. Cryopreservation of shoot-tips by droplet vitrification applicable to all taro (Colocasia exculenta var. esculenta) accessions. Plant Cell Tissue and Organ Culture 92:107-111.
Takagi H, Thinh NT, Islam OM and Senboku T. 1997, Cryopreservation of in vitro-grown shoot tips of taro (Colocasia esculenta (L.) Schott) by vitrification. 1. Investigation of basic conditions of the vitrification procedure. Plant Cell Reports 16:594-599.
Thinh NT, Takagi H, Yashima S. 1999. Cryopreservation of in vitro-grown shoot tips of banana (Musa spp.) by vitrification method. CryoLetters 20 (3):163-174.
More Articles...
Subcategories
-
main
- Article Count:
- 2
-
Registration of Musa
Registration and information systems - Importance and uses
Registration is the first step after acquisition of a sample in any genebank. Collections in genebanks are the genetic base for current and future breeding programs and a source of safety material for distribution to researchers and other users. It is essential that samples are all properly documented from the moment they enter a genebank as well as through all subsequent genebank operations.
A step by step guideline can be seen by clicking here.How should it be done?
Information systems
An information management system must be created in each genebank. This database must be searchable by the genebank curators and staff for specific information through a range of queries.
Numbering and labelling systems
Consecutive alpha numeric or numeric codes must be used for each new accession acquired. This code must be linked to all subsequent information about this sample: passport data, designation status and taxonomic information. The information system must keep a record of genebank operation data, including storage location, stocks, monitoring, health tests and the distribution status. The same system must also manage germplasm orders, shipment related information and files genebanks ‘contacts’ information.
Bar-coding is a useful tool that can compliment a genebank information system.
Extra samples
A separate subset of materials should be kept after registration to be regenerated under greenhouse conditions for harvesting leaf samples that can be processed for DNA/lyophilized leaf bank. These banked leaf materials can serve as a voucher for the germplasm stored in the active and base collections and samples can be made available to users for research in gene discovery and function, marker development and detailed genotypic characterisation. Method to be detailed by INIBAP
Musa Germplasm Information Systems (MGIS)
In 1997, INIBAP laid the basis for a global information system for Musa through the release of MGIS. The aim of the system was to enhance knowledge on Musa diversity, to help rationalizing conservation and to improve the use of banana genetic resources though a facilitated access to comprehensive information.
In 2005, the MGIS database contained key information, including passport data, botanical classification, morpho-taxonomic descriptors and characteristics such as agronomic traits, disease resistance, stress tolerance, biochemical or molecular genetic markers, and plant photographs as well as GIS information on 5188 accessions managed in 18 banana collections (link to the list of collections) around the world making it the most extensive source of information on banana genetic resources.
The database is publicly accessible through the internet at MGIS homepage.htm or at www.mgis.grinfo.net. This global database can be queried on the identity, origin, characteristics and distribution of the individual accessions in the collections. This allows curators of the participant institutions worldwide to share and compare their data. The database is also particularly helpful for various germplasm users namely breeders, researchers and farmer communities, in locating alternative sources of banana germplasm and identifying the most appropriate accessions with particular traits of interest.Homepage of the MGIS website (click on the picture if you wish to go there now)
Further reading:
Van den houwe et al., 2005 (The management of banana (Musa spp.) genetic resources at the IPGRI/INIBAP gene bank: the conservation and documentation status
Calles et al., 2003 (Best Practices for Genebank Management)- Article Count:
- 1
-
Conservation of musa
- Article Count:
- 4
-
In vitro bank for banana
- Article Count:
- 4
-
Cryopreservation for musa
- Article Count:
- 3
-
In the field for musa
- Article Count:
- 3
-
Safety duplication of musa
No. of samples (tubes) per line or cultivar
Size of container
Kind of medium
Amount of medium
Labeling
Placement of label
Bioversity - written directly on cryotubes using pencil
IITA - higher halfLabeling material
Bioversity - not applicable
IITA - marker on parafilm for tubes, tape on polyethylene bagsLabel information
Bioversity - accession ID, freezing date, experiment number
IITA - accession number, line number and date of last introduction
Viability testing
Conditions or timing when the test is conducted
After being one hour in liquid nitrogenNo. of samples for testing
Bioversity - 3 cryotubes per accession (experiment)
IITA – 1-5 seedlingsCriterion for long-term storage
At least 95% certainty that a minimum of 1 plant can be regenerated per experimentTransport
Type of container
Bioversity - dry shipper
IITA – plastic boxesMethod and duration
Bioversity - Air courier or hand carried
IITA - car (4x4) 5 to 6 hours driveConditions
Bioversity - Frozen in liquid nitrogen
IITA - ambientFrequency of shipment
Bioversity - Initially 3 to 4 x a year; once a year thereafter
IITA – 3 to 4 months
Genebank for safety storage
Bioversity - IRD, France & KULeuven, Belgium
IITA - IITA Cotonou, Benin
Storage
Type of storage
Bioversity – Cryopreservation in liquid nitrogen tank
IITA – Tissue culture slow growthType of container
Bioversity - 2mL cryotubes
IITA - Polyethylene bags (13 x 1.3 cm)Temperature (in degrees Celcius)
Bioversity – (-196 oC)
IITA – 18oCLife expectancy of clones
Bioversity - Indefinite
IITA – 4-6 monthsBack-up generator
Bioversity - none
IITA - noneOther features
Availability of liquid nitrogen alarm systemData arrangements
Bioversity - Germplasm ID, inventory of box content sent with samples
IITA - mport permit stating list of accession transferred, endorsed by PQS prior to departure Report at boarder PQS office
Provision for replacement of germplasm
Bioversity - If less than 95% certainty that one minimum plant can be regenerated per experimentProvision for return of germplasm
Bioversity - Loss of samples from LTS at Bioversity ITC; sample provided by the duplication site on Bioversity's requests on a 6 month written notice
IITA - Repatriation permit to ask when needed- Article Count:
- 1