CGKB News and events Cryopreservation for musa
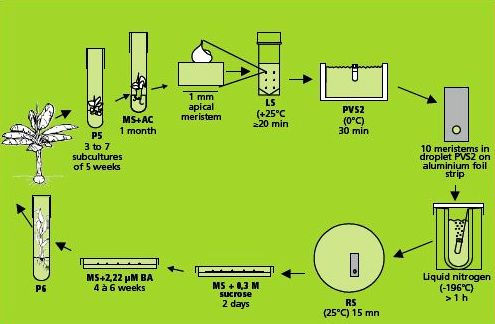
Cryopreservation of banana apical meristems
.jpg) View the full publication on Cryopreservation of Musa germplasm by clicking on the icon above (1.35 MB) |
Contributors to this page: Bioversity International, Belgium (Bart Panis).
The content of this page was extracted from Panis B. 2009. Cryopreservation of Musa germplasm: 2nd edition. Technical guidelines No. 9 (F. Engelmann and E. Benson, eds). Bioversity International, Montpellier, France.
Cryopreservation of in vitro grown shoot tips of banana through vitrification was originally reported by Thinh and co-workers (Thinh et al. 1999). Using this method, regeneration percentages were often low and unpredictable. Therefore, the technique was further improved and adapted at KULeuven to make it applicable to a wide variety of cultivars (Panis et al. 2005).
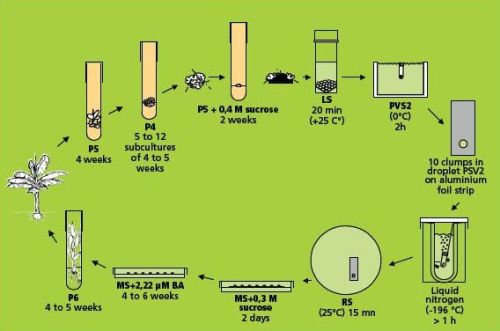
This method is illustrated in the figure below.
|
Cryopreservation of individual meristems (photo: KULeuven/Bioversity) |
Preparation of plant materials
|
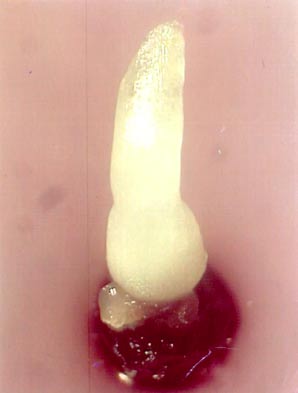
Production of robust, rooted in vitro plants of banana cv. ‘Williams’ (photo: KULeuven/Bioversity) |
Preparation of in vitro plantlets
All accessions were obtained from the Bioversity Musa germplasm in vitro collection (KULeuven, Belgium). This collection contains edible banana cultivars, as well as wild relatives.
- Shoot cultures are grown in 25x150 ml test tubes on 25 ml p5 medium. P5 medium contains semi-solid Murashige and Skoog (MS) medium supplemented with 30 g/L sucrose, 10 μM BA and 1 μM IAA and solidified with 2 g/L gelrite (Banerjee and de Langhe 1985). They are cultured at 25±2°C under continuous 50 μE m-2 s-1 illumination provided by 36W Osram cool-white fluorescent tubes. The pH is adjusted to 5.8 prior to autoclaving.
- From these multiplying cultures, shoots of 3-5 cm in length are separated and transferred to rooting medium in test tubes. The rooting medium has the same composition as p5, but it is devoid of plant growth regulators and supplemented with 0.5 g/L active charcoal.
- After one month, robust and well rooted in vitro plants are obtained with a corm diameter of 5-8 mm that forms an appropriate source for the excision of apical meristems (see photo).
Dissection and selection of apical meristems
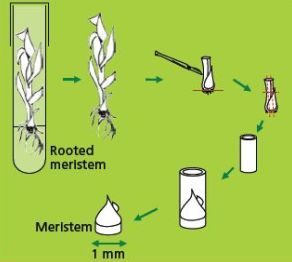
Like many other monocots, banana apical meristems are tightly covered with several layers of whitish, tubular, immature leaves.
- Individual apical meristems are excised under a binocular microscope. Leaves are removed one by one until the apical dome is visible, but still partially covered by 1-2 young leaf primordia (see figure and photo below).
- The leaf base (corm tissue) is 1 mm in diameter. In order to excise corm pieces of exactly this size, a millimetre grid graph paper is placed underneath the transparent sterile plastic Petri dish in which the meristems are excised.
- The dissected meristems are transferred into the loading solution (in the dark at room temperature). Tips that are slightly damaged or are not in the correct stage (i.e. the meristem is too much or too little covered by leaf primordia) are excluded from cryopreservation.
A skillful and trained technician can isolate a maximum of ten meristems an hour (roughly, six minutes/isolated meristem).
|
Illustration of meristem isolation. Leaves are removed one by one until the apical dome is visible but still partially covered by 1-2 young leaf primordia |
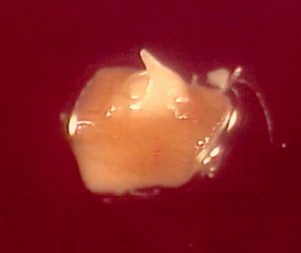
Partly-covered apical meristems of banana cv. ‘Williams’ (photo: KULeuven/Bioversity)
|
Cryopreservation through droplet vitrification
Loading, dehydration and rapid freezing
|
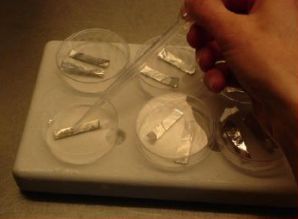
Transfer of meristems to a droplet of PVS2 solution (of about 15 μl) on a strip of aluminium foil (5x20 mm) with a 2 ml plastic Pasteur pipette (photo: KULeuven/Bioversity) |
- The filter sterilised loading solution contains 2 M glycerol and 0.4 M (= 136.8 g/L) sucrose dissolved in MS medium (pH 5.8). The excised meristems are left in the loading solution in a 20 ml plastic vessel until all of them are dissected. The exposure time thus varies between 20 min and 5 hrs. Previous research has shown that regrowth of banana meristems is not influenced by loading solution exposure time (Panis et al. 2005). Although the precise mechanism of loading is not yet fully understood, it has been proven for different plant species that loading can dramatically enhance the tolerance of isolated meristems to dehydration by the vitrification solution (Matsumoto et al. 1994, Takagi et al. 1997).
- After loading, the solution is replaced by ice-cooled PVS2 solution. The PVS2 solution consists of 30% (w/v) (3.26 M) glycerol, 15% (w/v) (2.42 M) ethylene glycol (EG), 15% (w/v) (1.9 M) DMSO and 0.4 M (= 136.8 g/L) sucrose (Sakai et al. 1990).
- All these compounds are dissolved in MS medium, pH adjusted to 5.8, followed by filter sterilization. The meristems are subjected to the PVS2 solution for 30-40 min. at 0°C.
- Five min. before the end of the treatment, ten meristems are transferred individually to one droplet of PVS2 solution (of about 15 μl) on a strip of aluminium foil (5x20 mm) with a 2 ml plastic Pasteur pipette (see photo).
- To keep the temperature of the strip around 0°C during the manipulations, the aluminium strip is placed in a plastic Petri dish placed on top of a frozen cooling element. After the PVS2 treatment, the aluminium strip is plunged into liquid nitrogen with a fine forceps.
- For permanent cryostorage, the frozen foil is quickly transferred to a 2 ml cryotube filled with liquid nitrogen and closed.
Storage, re-warming and unloading
- The meristems are kept in liquid nitrogen for at least 20 min. For re-warming, aluminium foil strips are rinsed in 10 ml unloading solution in a small Petri dish at room temperature. After a few seconds, meristems are released from the strip of aluminium foil and kept for another 15 min in the unloading solution. As such, the toxic PVS2 solution is removed whilst re-warming and replaced by a less-toxic unloading solution. The unloading solution consists of 1.2 M (= 410.4 g/L) sucrose dissolved in MS medium (pH 5.8).
Recovery
- Unloaded meristems are placed onto two sterile filter papers on top of semi-solid hormone-free MS medium containing 0.3 M (=102.6 g/L) sucrose.
- After two days, the meristems are transferred onto regeneration medium without filter papers. The first week of culture always takes place in the dark.
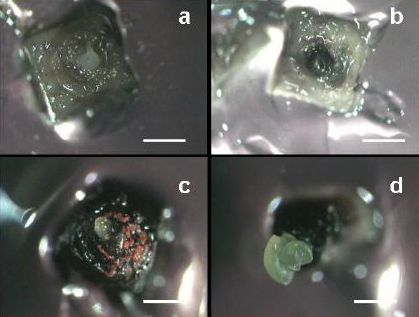
Four to six weeks following cryopreservation, four types of reactions can be distinguished:
(i) white shoot tips resulting from an immediate death of the tissue without blackening.
(ii) completely or partially black shoot tips, indicating that there was enzymatic reaction following cryopreservation (production and oxidation of polyphenols).
(iii) unorganized callus growth, representing the outgrowth of small isolated areas of the apical dome and/or primordial tissues.
(iv) shoot tip regeneration resulting from the survival of a substantial part of the apical dome (see figures a-d).
 |
|
|
Reaction of apical meristems towards cryopreservation 30 days after re-warming (a) No growth; the meristem remains white; (b) Blackening without further growth; apical dome reacts by formation of polyphenolic compounds that oxidize; (c) Callus formation; watery, non-morphogenic callus; and (d) Shoot regeneration (bar = 600 μm)(photo: KULeuven/Bioversity) |
One month after re-warming, a 0.5 cm long shoot can be observed (see photo). Calluses never produced shoots.
|
Recovered shoots from cryopreserved apical meristems of banana cv. ’Williams’ 1 month after re-warming |
Discussion and perspectives
Different Musa genotypes have been cryopreserved using this protocol (Thinh et al. 1999, Panis et al. 2005). Post re-warming regeneration percentages range between 20% and 85% (with an average of 53%). These recovery percentages are relatively genotype independent. It was, however, observed that genotypes with more B-genome survive significantly better than those with solely the A-genome (Panis et al. 2005). Microscopic observation of the recovery of cryopreserved meristems (Helliot et al. 2003) has revealed that:
(i) the whole dome of the isolated meristem withstands exposure to liquid nitrogen.
(ii) no regenerable post cryopreservation callus is formed. Therefore, it would appear that somaclonal variation is unlikely to occur.
The main limitations to this procedure are as follows:
- Post cryopreservation viability/regeneration percentages vary depending on the operator. Considerable experience in the dissection of tiny and fragile banana apical meristems is required before this cryopreservation protocol can be applied (using this method only 60 meristems can be excised and cryopreserved in one day).
- Low regrowth may be due to low quality meristems (tips that are slightly damaged or are not at the correct stage or too much or too little covered by leaf primordia). The quality of the donor plants (more light, fewer plantlets in containers) should be carefully monitored.
- Using the droplet vitrification method, some researchers have shown some concern with respect to the effects of the direct contact of liquid nitrogen with the plant material. Supposed problems like contamination and loss of material were, however, never encountered.
This droplet vitrification protocol was recently successfully applied to a wide range of plant species such as potato, ulluco, sweet potato, chicory, strawberry, taro, Pelargonium L., date palm, thyme, olive, Byrsonima, snowdrop, apple and hop and might thus be considered as the first generally applicable protocol (Panta et al. 2006, Gallard et al. 2008, Sant et al. 2008, Feki et al. 2011, Sánchez-Romero and Panis 2009, Marco-Medina et al. 2010, Condello et al. 2011, Maslanka et al. 2013, Coutinho Silva et al. 2013).
References and further reading
Banerjee N, De Langhe E. 1985. A tissue culture technique for rapid clonal propagation and storage under minimal growth conditions of Musa (banana and plantain). Plant Cell Reports 4:351-154.
Condello E, Caboni E, André E, Piette B, Druart P, Swennen R, Panis B. 2011. Cryopreservation of apple in vitro axillary buds using droplet-vitrification. CryoLetters 32:175-185.
Coutinho Silva L, Paiva R, Swennen R, André E, Panis B. 2013. Shoot-tips cryopreservation by droplet vitrification of Byrsonima intermedia A. juss.: a wooden tropical and medicinal plant species from Brazilian Cerrado. CryoLetters 34: 338-348.
Feki L, Bouaziz N, Sahnoun N, Swennen R, Drira N, Panis B. 2011. Palm cryobanking. CryoLetters 32:451-462.
Gallard A, Panis B, Dorion N, Swennen R, Grapin A. 2008. Cryopreservation of Pelargnonium apices by droplet-vitrifications. CryoLetters 29 (3):243-251.
Helliot B, Swennen R, PouMay Y, Frison E, Lepoivre P, Panis B. 2003. Ultrastructural changes associated with cryopreservation of banana (Musa spp.) highly proliferating meristems. Plant Cell Reports 21:690-698.
Marco-Medina A, Casas JL, Swennen R, Panis B. 2010. Cryopreservation of Thymus moroderi by droplet vitrification. CryoLetters.
31 (1):14-23.
Maslanka M, Panis B, Bach A. 2013. Cryopreservation of Galanthus elwesii Hook. apical meristems by droplet vitrification. Cryo Letters, 34: 1-9.
Matsumoto T, Sakai A, Yamada K. 1994. Cryopreservation of in vitro-grown apical meristems of wasabi (Wasabi japonica) by vitrification and subsequent high plant regeneration. Plant Cell Reports 13:442-446.
Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco cell cultures. Physiologia Plantarum 15:473–497.
Panis B. 2009. Cryopreservation of Musa germplasm: 2nd edition. Technical Guidelines No. 9 (F. Engelmann and E. Benson, eds). Bioversity International, Montpellier, France. Available here.
Panis B, Piette B, Swennen R. 2005. Droplet vitrification of apical meristems: a cryopreservation protocol applicable to all Musaceae. Plant Science 168:45-55.
Panta A, Panis B, Ynouye C, Criel B, Swennen R, Roca W. 2006. Improvement of potato cryopreservation for the long-term conservation of Andean landraces at CIP. 43rd Meeting of the Society for Cryobiology in association with the Society for Low Temperature Biology. Hamburg, Germany, 24-27 July 2006.
Sakai A, Kobayashi S, Oiyama I. 1990. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Reports 9:30-33.
Sánchez-Romero C, Swennen R, Panis B. 2009. Cryopreservation of olive embryogenic cultures. CryoLetters 30 (5):359-372.
Sant R, Panis B, Taylor M, Tyagi A. 2008. Cryopreservation of shoot-tips by droplet vitrification applicable to all taro (Colocasia exculenta var. esculenta) accessions. Plant Cell Tissue and Organ Culture 92:107-111.
Takagi H, Thinh NT, Islam OM and Senboku T. 1997, Cryopreservation of in vitro-grown shoot tips of taro (Colocasia esculenta (L.) Schott) by vitrification. 1. Investigation of basic conditions of the vitrification procedure. Plant Cell Reports 16:594-599.
Thinh NT, Takagi H, Yashima S. 1999. Cryopreservation of in vitro-grown shoot tips of banana (Musa spp.) by vitrification method. CryoLetters 20 (3):163-174.
Cryopreservation of banana meristem clusters (cauliflower-like structures)
.jpg) View the full publication on Cryopreservation of Musa germplasm by clicking on the icon above (1.35 MB) |
Contributors to this page: Bioversity International, Belgium (Bart Panis).
The content of this page was extracted from Panis B. 2009. Cryopreservation of Musa germplasm: 2nd edition. Technical guidelines No.9 (F. Engelmann and E. Benson, eds). Bioversity International, Montpellier, France.
A second type of regenerative meristematic tissue in banana which has been successfully cryopreserved is the highly proliferating (sometimes called cauliflower-like) meristem clusters. This tissue type was originally produced as a starting material to initiate embryogenic cell suspension cultures in bananas (Dhed’a et al. 1991, Strosse et al. 2006, Schoofs 1997).
Two cryopreservation techniques applied to highly proliferating ‘cauliflower-like’ meristem clusters are described below:
Contents: |
Preparation of plant materials
|
Meristem cultures of banana cultivars Nakitengwa (AAA highland banana), Williams (AAA group) and Bluggoe (ABB group) on (left) p5 medium (containing 10μM BA), and (right) p4 medium (containing 100μM BA)(bar = 2 cm) (photo: KULeuven/Bioversity) |
Production of ‘cauliflower-like’ meristem clusters
To produce this kind of material in all Musa accessions, meristem cultures are transferred to a medium containing a high concentration of BA (P4 medium, see Appendix 1). Every one to two months, the material is subcultured and small clumps of ‘cauliflower-like’ meristems are selected and transferred to fresh medium (Strosse et al. 2006). The high BA concentration (up to 100 μM) in the P4 medium suppresses outgrowth of meristems, thereby favouring the formation of numerous white apical domes (see photo on the right). Repeated subculturing is necessary and can take
4-12 months.
Preculture of meristem clumps
- Following the appearance of ‘cauliflower-like’ clusters, and four weeks after the last subculture, white meristematic clumps of about 4mm diameter, each containing at least four apical domes, are excised and transferred onto preculture medium (P5 + 0.4 M (= 136.8 g/L) sucrose) for four weeks. They are cultured at 25°C ± 2°C in darkness.
Discussion and perspectives
The quality of ‘cauliflower-like’ meristem clumps may be too poor for use in cryopreservation experiments (meristematic tissue versus corm tissue is too low and/or the explant shows too much blackening). This can be due to the fact that the cultivar belongs to a ‘difficult’ genomic group (for example East African highland bananas and many plantains). Previously, proliferation was only obtained by using BA at extremely high, nearly toxic, concentrations (100 μM). Prolonged culture on 100 μM BA containing media, therefore, often results in a quality decrease (loss of the typical ‘cauliflower-like’ characteristics) of the cultures. Recently, the use of alternative cytokinins like thidiazuron (TDZ) at lower concentration (1 μM) proved to increase proliferation rates (Strosse et al. 2008).
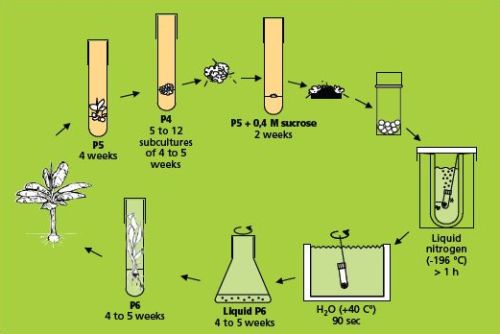
The simple freezing method (which involves sucrose preculture)
This method (Panis et al. 1996) is illustrated below.
|
The simple freezing method (figure: KULeuven/Bioversity) |
Cryopreservation
|
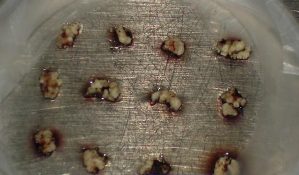
Precultured meristem clumps of "Musa schizocarpa"
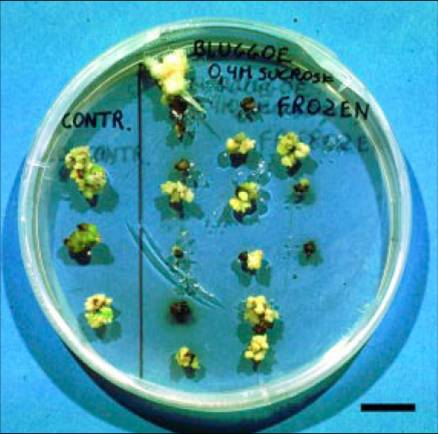
Petri dish containing control (left) and frozen (right) meristematic clumps of the cv. Bluggoe (ABB group), eight weeks after cryopreservation (bar = 1 cm) |
- Small white meristematic clumps weighing 5-15 mg (2-3 mm diameter), containing three to six meristematic domes, are excised from the precultured clumps. Brown tissues are removed and only the healthiest parts, as indicated by a white-yellowish colour, are retained.
- The clumps are transferred to sterile cryotubes (2 ml) without any liquid solution and plunged directly into a Dewar flask containing liquid nitrogen. Each cryotube contains 7-10 clumps. At this stage, samples can be stored for the long term by transferring the cryotubes to the liquid nitrogen tank, ensuring that their transfer from one container to the other takes place rapidly (within a few seconds), thereby preventing slow lethal re-warming of samples.
Re-warming and recovery
- After storage, rapid thawing takes place by stirring the frozen cryotube in a water bath at 40°C for 90 seconds.
-
Regeneration of the frozen meristems can be carried out in two different ways:
- Meristems are transferred to 9 cm Petri dishes containing semi-solid regeneration medium (P6) and sealed with parafilm.
- Alternatively, regeneration can be executed in liquid medium. Thawed meristems are transferred to 100 ml Erlenmeyer flasks containing 30 ml of liquid regeneration medium (P6 without solidifying agent) and placed on a rotary shaker at 70 rpm.
- After one week of culture in dark conditions, Petri dishes and flasks are transferred to continuous light at 50 µE m-2 s-1. Cultures are kept at all times at 25°C ± 2°C.
- Three weeks after transferring to the regeneration medium, frozen meristem regrowth is determined under a binocular microscope. Two types of surviving tissues are distinguished, i.e. shoots and non-regenerating callus. All calluses are systematically discarded and only recovered shoots (see photo on the right) are transferred to test tubes with regeneration medium to promote further development of whole plants. As soon as rooted plants are sufficiently developed, they are planted in soil.
Discussion and perspectives
The simple freezing protocol was applied to 36 banana cultivars belonging to 8 genomic groups (Panis et al. 2002). The results were extremely genotype dependent. Best results (up to 70% regrowth) have been obtained with the ABB cultivars like Bluggoe, Cachaco and Monthan. Intermediate results (around 25% regrowth) were reached with AAA dessert and AAB bananas. AAB plantains and diploids generally respond poorly. For all cultivars under investigation belonging to these genomic groups, plants were regenerated and grown in the greenhouse. However, most of the AAA Highland bananas were not able to withstand simple freezing.
With regard to this simple freezing method, blackening, due to the oxidation of polyphenols is often observed when re-warmed meristems are placed on semi-solid medium. This can cause cytotoxic effects and may also result in the recovering clumps being surrounded by an impermeable layer, thereby preventing nutrient uptake for further outgrowth. One method to overcome this problem is to use liquid regeneration media in order to dilute the released polyphenols. This resulted in regeneration percentages that were up to 20% higher
The droplet vitrification of ‘cauliflower-like’ meristem clusters method (combining simple freezing with droplet-vitrification)
This method (Panis et al. 2000, Agrawal et al. 2004) is illustrated in the figure below. Loading, dehydration, rapid freezing, storage, re-warming and unloading are almost identical to the droplet vitrification described in the page on Cryopreservation of banana apical meristems. Therefore, only the essential (and differential) steps are indicated in detail below.
|
Droplet vitrification protocol of meristem clumps (figure: KULeuven/Bioversity) |
Loading, dehydration and rapid freezing
|
Meristem clumps in a droplet of PVS2 solution (of about 15 μl) on a strip of aluminium foil (5x20 mm) |
- The excised sucrose precultured meristem clumps are left in the loading solution (see Appendix 1) in a 20 ml plastic vessel until all of them are dissected. The exposure time thus varies between 20 min. and 3 hrs.
- After loading, the solution is replaced by ice-cooled PVS2 solution (see Appendix 1). The meristem clumps are subjected to the PVS2 solution for 2 hrs. at 0°C. Five min before the end of the treatment, about ten meristem clumps are transferred to one droplet of PVS2 solution on a strip of aluminium foil (5x20 mm) with a forceps and a 2 ml plastic Pasteur pipette (see photo).
- To keep the temperature of the strip around 0°C during the manipulations, it is placed in a plastic Petri dish placed on top of a frozen cooling element. After the PVS2 treatment, the aluminium strip is plunged into liquid nitrogen with a fine forceps. For permanent cryostorage, the frozen foil is quickly transferred to a 2 ml cryotube filled with liquid nitrogen and closed.
Storage, re-warming and unloading
- The meristem clumps are kept in liquid nitrogen for at least 20 min. For re-warming, aluminium foil strips are plunged in 10 ml unloading solution (see Appendix 1) in a small Petri dish at room temperature. After a few seconds, meristem clumps are released from the aluminium foil and kept for another 15 min in the unloading solution. As such, the toxic PVS2 solution is removed whilst re-warming and replaced by a less-toxic unloading solution.
Recovery
- Frozen meristem clumps are taken from the unloading solution and placed in 9 cm plastic Petri dishes on two sterile filter papers on top of about 25 ml semi-solid hormone-free MS medium containing 0.3 M (= 102.6 g/L) sucrose.
- After two days, the filter paper is removed and the meristem clumps are transferred to Petri dishes with MS medium supplemented with 2.22 μM BA. The first week of culture always takes place in the dark.
- After a maximum of six weeks, meristem clumps are transferred to test tubes with P6 medium for further development of whole plants (see photos below).
 |
 |
|
Regenerating shoots from one control and three frozen proliferating meristems of the cultivars ‘Kisubi’ (AB group) ‘Dominico Harton’ (AAB plantain), ‘Bluggoe’ (AAB group) and ‘Williams’ (AAA group) three months after cryopreservation (photos: KULeuven/Bioversity) |
|
Discussion and perspectives
It has been found that post re-warming regrowth percentages of sugar pregrown meristems are higher compared to those grown on normal P4 medium. Sucrose preculture seems to increase tolerance of meristems not only towards the PVS2 solution but also towards the damaging events taking place during the cooling process. When comparing the results from the droplet vitrification of ‘cauliflower-like’ meristem clusters with those obtained using the simple freezing method for the same cultivar, an increase in viability percentages for almost all cultivars is observed. The increase in post-thaw regeneration for the ABB bananas is limited. Recovery remains between 50 and 70%. For AAA dessert and AAB bananas, the increase of regeneration percentages amount to 30-50%, while for plantains 20-30% is reached. AAA Highland bananas which proved to be recalcitrant towards cryopreservation using simple freezing give 0-20% survival using the droplet vitrification method.
For most plant species, optimal dehydration of meristematic tissues with PVS2 is obtained after 10-30 min. at room temperature (Takagi 2000). Among the exceptions are shoot apices of sweet potato and pineapple which need to be treated with PVS2 for 100 min. and 7 hrs., respectively (Plessis and Steponkus 1996, Gonzalez-Arnao et al. 1998). The duration of this treatment has to be optimized case by case since enough dehydration must take place to avoid the formation of lethal ice crystals during freezing. At the same time care has to be taken to prevent the treatment with the potentially toxic solution from irreversibly damaging the tissue. In the case of sucrose precultured proliferating banana cultivars, it has been observed that optimal post re-warming regeneration percentages are generally obtained after a 2 or 2.5 hr. PVS2 treatment. Survival after 3 hrs for most cultivars is considerably lower, probably due to the toxicity of this highly concentrated solution.
References and further reading
Agrawal A, Swennen R, Panis B. 2004. A comparison of four methods for cryopreservation of meristems in banana (Musa spp.). CryoLetters 25:101-110.
Dhed’a D, Dumortier F, Panis B, Vuylsteke D, De Langhe E. 1991. Plant regeneration in cell suspension cultures of the cooking banana cv. ‘Bluggoe’ (Musa spp., ABB group). Fruits 46 (2):125-135.
Gonzalez-Arnao MT, Ravelo MM, Villavicencio CU, Montero MM, Engelmann F. 1998. Cryopreservation of pineapple (Ananas comosus) apices, CryoLetters 19: 375-382.
Panis B. 2009. Cryopreservation of Musa germplasm: 2nd edition. Technical Guidelines No. 9 (F. Engelmann and E. Benson, eds). Bioversity International, Montpellier, France. Available here.
Panis B, Schoofs H, Thinh NT, Swennen R. 2000. Cryopreservation of proliferating meristem cultures of banana. In: Engelmann F, Takagi H, editors. Cryopreservation of tropical plant germplasm. Current research progress and application. Japan International Research Center for Agricultural Sciences, Tsukuba, Japan / International Plant Genetic Resources Institute, Rome, Italy. pp. 238-243.
Panis B, Strosse H, Van den Hende S, Swennen R. 2002. Sucrose preculture to simplify cryopreservation of banana meristem cultures. CryoLetters 23:375-384.
Panis B, Totté N, Van Nimmen K, Withers LA, Swennen R. 1996. Cryopreservation of banana (Musa spp.) meristem cultures after preculture on sucrose. Plant Science 121:95-106.
Plessis P, Steponkus PL. 1996. Effect of preculture in a sucrose-containing medium on the sugar content of potato shoot-tips, Cryobiology 33: 654-655.
Schoofs H. 1997. The origin of embryogenic cells in Musa. Dissertationes de Agricultura 330. Katholieke Universiteit Leuven, Belgium. 257 pp.
Strosse H, Schoofs H, Panis B, André E, Reyniers K, Swennen R. 2006. Development of embryogenic cell suspensions from shoot meristematic tissue in bananas and plantains (Musa spp.). Plant Science 170:104-112.
Strosse H, André E, Sági L, Swennen R, Panis B. 2008. Adventitious shoot formation is not inherent to micropropagation of banana as it is in maize. Plant Cell Tissue and Organ Culture 95:321-332.
Takagi H. 2000, Recent developments in cryopreservation of shoot apices of tropical species. In: Engelmann F, Takagi H, editors. Cryopreservation of tropical plant germplasm. Current research progress and application. IPGRI, Rome, Italy. pp. 178-193.
Cryo storage for banana genetic resources
.jpg)
View the full publication on Cryopreservation of Musa germplasm by clicking on the icon above |
Contributors to this page: Bioversity International, Belgium (Bart Panis).
The contents of these Cryo pages were extracted from Panis B. 2009. Cryopreservation of Musa germplasm: 2nd edition. Technical guidelines No.9 (F. Engelmann and E. Benson, eds). Bioversity International, Montpellier, France.
Introduction
In the last 20 years, several new cryopreservation procedures like vitrification, encapsulation-dehydration, preculture-dehydration, and encapsulation vitrification have been established, which are all based on vitrification. Vitrification can be defined as the transition of water directly from the liquid phase into an amorphous phase or glass, whilst avoiding the formation of crystalline ice. Cryopreservation protocols based on vitrification techniques have been developed for different vegetatively propagated crops, including banana (Sakai and Engelmann 2007).
When cryo banks are used
|
‘Cryopreserving’ samples or placing samples into a state of suspended animation in liquid nitrogen (photo: Bioversity) |
|
|
A box containing cryotubes (photo: Bioversity) |
Research on cryopreservation of Musa was initiated in the 1990ties at Bioversity International Transit Center (ITC, previously INIBAP). The main objective was to:
- Overcome some of the limitations of in vitro maintenance (labour-intensive subculturing, potential for fungal and bacterial contaminants, as well as the risk for somaclonal variation).
- To ensure the safe long-term conservation of Musa genetic resources.
Current situation
Bioversity-supported research at KULeuven has resulted in the development of three cryopreservation protocols suitable for the long-term storage of meristem cultures of banana.
- The first method relies on rapid freezing of highly proliferating meristem cultures, precultured for two weeks on medium with 0.4 M (136.8 g/L) sucrose.
- The second method also uses highly proliferating meristem cultures that are precultured on sucrose but they receive an additional vitrification treatment.
- The third and most generally applicable protocol is the vitrification of apical meristems excised from rooted in vitro plants.
The labour requirement to cryopreserve accessions, as well as the post-re-warming regeneration percentages, depends on the cultivar and the method used (Panis et al. 2007).
The application of the aforementioned protocols has resulted thus far in the safe storage in liquid nitrogen of 882 accessions (situation September 2013) belonging to the different genome groups within the genus Musa.
Detection of endophytic bacteria
During normal meristem culture and storage under limited growth conditions, the presence of endogenous bacteria is rarely observed. If present, often such bacteria do not interfere with the growth of meristem cultures. However, as soon as meristems are subjected to cryopreservation, growth of endogenous bacteria becomes a problem. As the meristems begin to regrow following cryopreservation, endogenous bacteria present can develop into yellow or white colonies, which overgrow the recovering meristem.
- Prior to cryopreservation, cultures should always be screened for the presence of endophytic bacteria on a bacterial growth medium (BACT medium) containing 23 g/L Difco® Bacto nutrient broth, 10 g/L glucose and 5 g/L yeast extract (van den Houwe and Swennen 2000).
- The plates should be incubated for three weeks in the light at 28°C.
- Accessions with a positive response to bacterial screening should be discarded (Hamill et al. 2005, Thomas et al. 2008, Van den Houwe and Swennen 2000).
Method of cryopreservation at the ITC
- Currently, the droplet-vitrification of apical meristems, as well as the droplet-vitrification of ‘cauliflower-like’ meristem clusters, are both applied to the Musa collection.
- The simple freezing method is no longer applied for these purposes in view of the relatively low post-thaw regeneration percentages obtained for most genomic groups.
The table below compares the labour requirement for the two methods that are now applied.
Table: Comparison of labour requirement for two cryopreservation protocols.
|
Freezing method |
Labour time |
cvs/person |
Time needed |
Cultivars |
|
Vitrification of proliferating clumps |
30 to 40 |
59 to 44 |
12 to 13 |
ABB |
|
Vitrification of individual meristems |
60 to 70 |
29 to 25 |
8 to 9 |
AAAh bananas |
|
1: Calculated time needed to prepare the culture media and meristem cultures followed by cryopreservation. |
||||
- The most labour intensive method (vitrification of apical meristems excised from rooted in vitro plants) should only be applied to the cultivars which are difficult to cryopreserve with the other method (for example AAA highland bananas).
- For banana accessions belonging to the ABB group and AAB (non-plantain) bananas, the droplet-vitrification of proliferating clumps is always applied.
- The preferred method for Musa acuminata and the East African highland bananas is the droplet-vitrification of apical meristems.
- For all other accessions, the method of choice is based on (i) the proliferation degree of the meristem clumps that are obtained after three subculture cycles on a medium containing high cytokinin concentrations and (ii) survival of the proliferating meristem clumps after a preliminary cryopreservation trial.
To decide whether a cryopreservation experiment is successful or not, the mode of calculation developed by Dussert and coworkers (2003) should be used. These calculations have been applied to all ITC data.
An experiment is considered successful provided that the chance to regenerate at least one shoot from the stored material is more than 95%. This probability depends on:
(i) the number of explants stored in liquid nitrogen (in the long-term ranging between 30 and 50);
(ii) the number of explants re-warmed (ranging between 16 and 50); and
(iii) the post re-warming regeneration percentage (%).
An experiment leading to a lower probability level will not be considered successful, irrespective of its regeneration percentage. In such cases, the repetition is discarded and a new repetition will be executed.
A banana accession is considered “safely” preserved if three independent (and successful) experiments are completed. According to these requirements, ITC is currently storing 882 accessions belonging to 30 different genomic banana groups in liquid nitrogen (situation September 2013).
References and further reading
Agrawal A, Swennen R, Panis B. 2004. A comparison of four methods for cryopreservation of meristems in banana (Musa spp.). CryoLetters 25:101-110.
Hamill S, Wasmund K, Smith M, Eccleston K, McKay D. 2005. Endogenous bacterial isolated from banana meristems during tissue culture initiation: problems and potential. In: Benett IJ, Bunn E, Clarke H, McComb JA. editors. Contributing to a Sustainable Future. Proc. Australian Branch IAPTC & B. Perth, Western Australia, AU. pp.101-111.
Panis B. 2009. Cryopreservation of Musa germplasm: 2nd edition. Technical Guidelines No. 9 (F. Engelmann and E. Benson, eds). Bioversity International, Montpellier, France. Available here.
Panis B. 2008. Cryopreservation of Monocots. Chapter 11. In: Reed BM, editor. Plant Cryopreservation: A Practical Guide. Springer Science+Business Media, LLC. pp. 241-280.
Panis B, Helliot B, Strosse H, Remy S, Lepoivre P, Swennen R. 2005. Germplasm conservation, virus eradication and safe storage of transformation competent cultures. In: Chang WC, Drew R, editors. Banana: The importance of cryopreservation. Proceedings IInd IS on Biotech. of Trop. & Subtrop. Species. Acta Horticulturae 692. ISHS. pp. 51-59.
Panis B, Lambardi M. 2006. Status of cryopreservation technologies in plants (crops and forest trees). Chapter 6. In: Ruane J, Sonnino A, editors. The role of biotechnology in exploring and protecting agricultural genetic resources. Food and Agriculture Organization of the United Nations, Rome, Italy. pp. 61-78.
Panis B, Piette B, André E, Van den houwe I, Swennen R. 2011. Droplet vitrification: the first generic cryopreservation protocol for organized plant tissues? Acta Horticulturae 908:157-164
Panis B, Piette B, Swennen R. 2005. Droplet vitrification of apical meristems: a cryopreservation protocol applicable to all Musaceae. Plant Science 168:45-55.
Panis B, Strosse H, Helliot B, Lepoivre P, Remy S, Elsen A, De Waele D, Swennen R. 2002. Cryopreservation as a tool to store banana meristems and embryogenic cells, its nematodes and for virus eradication. 3rd International Symposium on Molecular and Cellular Biology of Bananas. Leuven, Belgium, 9-11 September 2002. 48 pp.
Panis B, Strosse H, Remy S, Sági L, Swennen R. 2004. Cryopreservation of banana tissues: support for germplasm conservation and banana improvement. In: Mohan Jain S, Swennen R, editors. Banana Improvement: Cellular, Molecular Biology, and Induced Mutations. Science Publishers Inc., Enfield, NH, USA:13-21. Available from: www.fao.org/docrep/007/ae216e/ae216e04.htm#bm04 Date accessed: 23 March 2010.
Panis B, Strosse H, Van den Hende S, Swennen R. 2002. Sucrose preculture to simplify cryopreservation of banana meristem cultures. CryoLetters 23:375-384.
Panis B, Thinh NT. 2001. Cryopreservation of Musa germplasm. INIBAP Technical Guidelines 5 (Escalant JV, Sharrock S, editors). International Network for the Improvement of Banana and Plantain, Montpellier, France. Available here.
Panis B, Totté N, Van Nimmen K, Withers LA, Swennen R. 1996. Cryopreservation of banana (Musa spp.) meristem cultures after preculture on sucrose. Plant Science 121:95-106.
Panis B, Van den Houwe I, Piette B, Swennen R. 2007. Cryopreservation of the banana germplasm collection at the International Transit Centre - Bioversity International. Advances in Horticultural Science 21 (4):235-238.
Sakai A, Engelmann F. 2007. Vitrification, encapsulation-vitrification and droplet-vitrification. CryoLetters 28:151-172.
Sakai A, Kobayashi S, Oiyama I. 1990. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Reports 9:30-33.
Strosse H, Schoofs H, Panis B, André E, Reyniers K, Swennen R. 2006. Development of embryogenic cell suspensions from shoot meristematic tissue in bananas and plantains (Musa spp.). Plant Science 170:104-112.
Swennen R, Markham R, Frison E. 2004. Applying biotechnology in banana and plantain: implications for developing countries. PBI Bulletin. Issue 2. Biotechnology and developing countries. The potential and the challenge. NRC-CNRC Plant Biotechnology Institute, Canada. pp. 22-27.
Thinh NT, Takagi H, Yashima S. 1999. Cryopreservation of in vitro-grown shoot tips of banana (Musa spp.) by vitrification method. CryoLetters 20 (3):163-174.
Thomas P, Swarna GK, Roy PK, Patil P. 2008. Identification of culturable and originally non-culturable endophytic bacteria isolated from shoot tip cultures of banana cv Grand Naine. Plant Cell Tissue and Organ Culture 93:55-63.
Van den Houwe I, Swennen R. 2000. Characterization and control of bacterial contaminants in in vitro cultures of banana (Musa spp.). Meeting: International Symposium on Methods and Markers for Quality Assurance in Micropropagation. Acta Horticulturae 530:69-79. An abstract and purchase of the publication is available from: www.actahort.org/books/530/530_6.htm. Date accessed: 23 March 2010.
Van den Houwe I, Panis B, Arnaud E, Markham R, Swennen R. 2006. The management of banana (Musa spp.) genetic resources at the IPGRI/INIBAP gene bank: the conservation and documentation status. In: Segers H, Desmet P, Baus E editors. Tropical biodiversity: science, data, conservation. Meeting: 3rd GBIF Science Symposium, Brussels, 18-19 April 2005. pp. 141-150. Available here. (8MB)
Wang QC, Panis B, Engelmann F, Lambardi M, Valkonen J. 2009. Cryotherapy of shoot tips: a technique for pathogen eradication to produce healthy planting materials and prepare healthy plant genetic resources for cryopreservation. Annals of Applied Biology 154:351-363.



 Cryopreservation for musa
Cryopreservation for musa