CGKB News and events Conservation of musa
Conservation of banana genetic resources
Contributors to this page: Bioversity International, France (Nicolas Roux, Anne Vezina, Max Ruas, Stéphanie Channeliere), Bioversity International, Belgium (Bart Panis, Ines Van den Houwe), Bioversity International, Ethiopia (Michael Bolton, Alexandra Jorge), CIRAD, France (Jean-Pierre Horry), Institute of Experimental Botany (Jaroslav Dolezel), IITA, Nigeria (Dominique Dumet, Badara Gueye).
Importance of banana conservation
 |
|
People in different parts of the world work together to conserve banana diversity |
Genetic improvement of varieties presents a potentially cost-effective mechanism to address current constraints in smallholder production, providing high-performing varieties that can adapt to diverse environments. These potential improvements are drawn from sources of resistance from wild and edible genotypes. However, new wild species and varieties are inadequately represented in collections. Threats posed by habitat destruction and replacement or loss of traditional cultivars intensify the urgency for collection and conservation efforts worldwide (INIBAP, 2006).
Recent banana projects have demonstrated a growing demand for increased diversity of cultivars, improved varieties not only within the research and breeding community but also among smallholder farmers and formal market systems. Supplying banana producers with a wider range of diversity can potentially broaden their livelihood options and can also enable family nutrition to be diversified (INIBAP, 2006).
Conservation of banana genetic resources is together with the other roots and tubers also subject of theme 1 “Conserving and accessing genetic resources” of the CGIAR Research Program on roots, rubers and bananas (CRP-RTB). Theme 1’s overall objective is to build upon the existing competencies of the four CGIAR Centers involved in clonal crop conservation to implement global conservation strategies for RTB crops in close collaboration with the Global Crop Diversity Trust (GCDT) and regional and national genebanks.
The Global Musa Genetic Resources Network, in short MusaNet, is a global collaborative framework for Musa genetic resources and a partnership of all key stakeholders, which aims at ensuring the long-term conservation on a cooperative basis.
Conservation methods
Banana (Musa) germplasm is usually seedless and options for its long-term conservation is therefore limited by the vegetative nature of the plant's reproductive system.
- Vegetative methods are the best and more widely used methods of banana conservation.
- Germplasm can be maintained as vegetatively maintained genotypes in fields or screenhouses (field banks), in tissue culture or via cryopreservation (in vitro).
- When the objective is solely to conserve the genes (but not specific combinations of genes), true seeds can be used instead, as long as seeds are produced. Seeds can probably also be conserved with cryo techniques.
- Genes can also be maintained for further use in the form of DNA (DNA banks or lyophilized leaves) or cryopreserved pollen.
Genebanks should have clear priorities on which type of germplasm should be collected, efficiently conserved and securely duplicated.
- Small collections may also give high priority to conserving all material.
- Larger collections should prioritize the conservation of local landraces.
- If core collections have been defined, these must be given the highest priority.
- Conservation of breeding lines and introductions should given lower priority than conservation of local material.
-
The conservation of local landraces must be particularly secured. Possible measures are:
- Use two separate field locations.
- Duplicate in vitro collections in separate locations.
- Maintain a field bank and an in vitro bank.
- Maintain a back up of the in vitro bank under cryopreserved conditions.
- Maintain a back up of the cryopreserved collection in a separate location as black box.
Major banana collections
It is estimated that there are at least 60 major national collections worldwide. Most banana collections manage their accessions in vivo (as full size plants) in the field. A survey carried out by INIBAP in 2006, reported that 25 field collections of banana hold more than 6000 accessions.
About 15 of the surveyed institutes (INIBAP, 2006) host in vitro collections containing slightly more than 2000 accessions, in addition to the Bioversity International Transit Centre (Bioversity ITC) that holds a further 1176 accessions (in vitro).
Cryopreservation methods were first developed at Bioversity ITC in 1996, and work to cryopreserve their entire banana collection started in 2003. The ITC has until now (situation September 2013) cryopreserved 882 accessions belonging to all Musa groups. This represents 63% of the accessions stored in vitro and is thus one of the world’s largest cryopreserved plant collections percentage wise. The National Research Centre for Banana (NRCB) in India and the International Institute of Tropical Agriculture (IITA) in Nigeria are also initiating working on some cryocpreservation methodologies.
Relevant banana collections with rich germplasm diversity or which provide relevant research, expertise, services or capacity building include: BPI, Philippines; CARBAP, Cameroon; CIRAD, France; DHIA, Honduras; DPI&F, Australia; EMBRAPA, Brazil; IEB, Czech Republic; IITA, Nigeria and Uganda; ITC, Belgium; University of Gembloux, Belgium; NARO, Uganda; NRCB, India; PPRI, South Africa; SPC, Fiji.
References and further reading
INIBAP. 2006. Global Conservation Strategy for Musa (Banana and Plantain). Available from: www.croptrust.org/documents/web/Musa-Strategy-FINAL-30Jan07.pdf. Date accessed: 23 March 2010.
MusaNet [online]. Homepage of the MusaNet portal. Available from: https://sites.google.com/a/cgxchange.org/musanet/home Accessed: 25 July 2011.
Panis B, Lambardi M. 2005. Status of cryopreservation technologies in plants (crops and forest trees). International Workshop on "The role of biotechnology for the characterisation and conservation of crop, forestry, animal and fishery genetic resources". Turin, Italy, 5-7 March 2005. pp. 43-54.
Van den Houwe I, Lepoivre P, Swennen R, Frison E, Sharrock S. 2003. The world banana heritage conserved in Belgium for the benefit of small-scale farmers in the Tropics. Plant Genetic Resources Newsletter 135:18-23.
Characterization of cultivated banana and wild types
|
Practicing characterization at a training course (photo: Bioversity) |
Contributors to this page: Bioversity International, France (Anne Vezina, Stéphanie Channeliere).
The Descriptors for Bananas, published in 1996 by Bioversity (then IPGRI and INIBAP), include the characterization descriptors developed by the French Agricultural Research Centre for International Development, CIRAD. This comprehensive list of 126 descriptors is intended to guide curators in deciding which ones are most useful to describe the material in their collection. It is not expected that all the descriptors will need to be recorded.
A set of Key access and utilization descriptors for banana have recently been developed by Bioversity International and an international advisory group (2009).
When to record descriptors
The best time to record the descriptors is when the fruit are well-filled, green-ripe or just turning yellow, and the rachis is at least 45 cm long. It should be noted however that, according to their nature, some of the descriptors will need to be recorded at a different time.
On a plant that loses its bracts, the optimal development stage to record descriptors can be confirmed by counting the number of nodes (the scars made by the fallen bracts) on the rachis, as shown below. Bracts fall off at the rate of one a day, revealing three parallel spirals. Counting 20 nodes on any of the three spirals means that plant flowered 60 days before. This is the point after which rapid change no longer occurs.
 |
 |
|
The photos above show the first ten nodes/scars of the first spiral, which continues on the back of the rachis. The spiral has to have at least 20 nodes to be at the optimal development stage for recording most of the descriptors. (photos: Bioversity) |
|
To ensure maximum expression of the characters to be recorded, the plants should be grown in an environment conducive to their development. The regeneration guidelines describe how to manage a field collection to obtain optimal growth conditions (see regeneration guidelines for banana).
Photographic descriptors
Photographic descriptors are useful to complement and corroborate the information obtained using the characterization descriptors. The photos can also be used by taxonomic experts to establish the identity of an accession. They should be taken when the characterization descriptors are recorded. Below are instructions on how to capture the photographic descriptors.
|
|
References and further reading
Bioversity International. 2009. Key access and utilization descriptors for banana genetic resources. Bioversity International, Rome, Italy. Available here.
IPGRI, INIBAP, CIRAD. 1996. Descriptors for Banana (Musa spp.). IPGRI, Rome, Italy; INIBAP, Montpellier, France; CIRAD, France. 55 pp. Available here.
Ploetz RC, Kepler AK, Daniells J, Nelson SC. 2007. Banana and plantain - an overview with emphasis on Pacific island cultivars. Available from: http://www.agroforestry.net/tti/Banana-plantain-overview.pdf. Date accessed: 23 March 2010.
Simmonds NW, Weatherup STC. 1990. Numerical taxonomy of the cultivated bananas. Tropical Agriculture 67 (1):90-92.
Simmonds NW, Weatherup STC. 1990. Numerical taxonomy of the wild bananas (Musa). New Phytologist 115 (3):567-571.
Simmonds NW, Shepherd K.1955. The taxonomy and origins of the cultivated bananas. Journal of the Linnean Society 55:302-312.
Field bank (regeneration) guidelines for banana
Contributors to this page: Bioversity International, France (Nicolas Roux), Bioversity International, Ethiopia (Michael Bolton, Alexandra Jorge); CIRAD, France (Jean-Pierre Horry, Tomekpe Kodjo); IITA, Nigeria (Dominique Dumet).
Field bank for banana
When are field banks used
|
Banana field collection (photo: Bioversity) |
Many important varieties of field, horticultural and forestry species, including banana, are either difficult or impossible to conserve as seeds (i.e. no seeds are formed or if formed, the seeds are recalcitrant) or to reproduce vegetatively. Hence they can be conserved as growing plants in field genebanks or in vitro (in tissue culture or cryo banks).
Banana stored in field genebanks have a lower risk of loosing genetic integrity (due to genetic drift) if the mother plants are maintained for many years and are readily available for study and use. However, bananas maintained in field genebanks are considerably more exposed to physical risks (climate, diseases, pests) and costs are higher for storage (labour, inputs and space) than for in vitro genebanks. This balance must be considered before taking the decision to establish a field genebank for banana, if other options are also possible.
Advantages of field genebanks
- Material can be evaluated and characterized while being conserved.
- Genotypes that commonly produce variants can be more easily identified and rogued out in the field than in vitro.
- Lower risk of loosing genetic integrity.
- Easy access for research, utilization and distribution.
- Establishment and maintenance of field genebanks requires limited technology.
Disadvantages of field genebanks
- Materials are susceptible to pests, diseases, adverse weather, theft and vandalism.
- Field genebanks require large areas of land, but even then genetic diversity is likely to be restricted.
- High maintenance costs (labour, inputs and space).
- Slow multiplication of material for distribution.
|
View regeneration guidelines in full (in PDF) |
Regeneration guidelines for banana
Note: These guidelines refer specifically to the regeneration of banana in field collections and include information on the establishment of a new field collection.
For regeneration or rejuvenation of banana in in vitro collections click here.
The information on this page was extracted from:
Tomekpe K. and Fondi E. 2008. Regeneration guidelines: banana. In: Dulloo M.E., Thormann I., Jorge M.A. and Hanson J., editors. Crop specific regeneration guidelines [CD-ROM]. CGIAR System-wide Genetic Resource Programme, Rome, Italy. 9 pp.
Before reading the regeneration details for this crop, please read the general introduction that gives the general guidelines to follow, by clicking here.
Introduction
Banana and plantain (Musa spp. L.) are giant monocotyledonous perennial herbs that thrive in the humid and subhumid tropics of low- and mid-altitude areas. They originate primarily from South-East Asia, with secondary centres of diversity in West and Central Africa (Plantain subgroup) and the East African highlands (Lujugira subgroup). They belong to the genus Musa, which comprises more than 1000 varieties in four sections: Australimusa, Callimusa, Rhodochlamys and Eumusa (Simmonds and Shepherd 1955). Most cultivated Musa belong to the Eumusa section and produce fruits that are a major commodity in international trade but are even more important as starchy staples in local food economies of many developing countries.
Most cultivars are derived from two species: Musa acuminata Colla (A genome) and Musa balbisiana Colla (B genome). Most edible banana are triploids (2n = 3x = 33), although there are a few diploid cultivars and even fewer tetraploid cultivars.
Bananas and plantains are also classified according to use (dessert, cooking, beer or processing/fibre) or mode of preparation of the fruit. Sweet bananas are eaten fresh or unprocessed but most bananas, and particularly plantains with orange pulp, are cooked. Cultivated bananas and plantains are parthenocarpic and are propagated vegetatively.
They are conserved in field collections as actively growing plants or in vitro as proliferating tissue. Wild relatives (normally non-parthenocarpic) are conserved in the same way. Bananas and plantains consist of an underground organ (corm) that bears roots, suckers or shoots and a pseudostem with leaves. The corm or rhizome is the true stem. Suckers develop initially as swollen buds from lateral meristems at leaf bases on the corm. A sucker has different developmental stages (Stover and Simmonds 1987): peeper, sword sucker and maiden sucker. The recommended stage for regeneration is the sword sucker (a sucker that is 50-150 cm in height with lanceolate leaves) followed by the peeper (a large green bud that has just emerged above ground). Among the followers or daughter plants, a sucker or ratoon is selected to succeed the mother plant. A group of suckers from a single parent is referred to as a stool or mat. A crop cycle is the time between planting and fruit harvest on the same mat. The second harvest from the mat is called the first ratoon crop (Gowen 1995).
 Description is an important step in regeneration: |
Choice of environment and planting
Climatic conditions
Banana grows well:
- In humid tropical lowlands between latitudes 20°N and 20°S.
- Between 100 m and 500 m altitude.
- Above 19°C mean minimum temperature (optimum average temperature is 27°C).
- In areas with >100 mm monthly rainfall (Robinson and de Villiers 2007).
However, banana can grow in a much wider range of climates within the tropics, with site selection mainly limited by soil type and rainfall.
Planting season
Planting can be done all year round if there is enough moisture. Planting should be done at the beginning of the rainy season.
Preparation for regeneration
When to regenerate
Regenerate field collections every four years to restore collections, as accumulated diseases and pests reduce plant vigour. Regeneration also allows maintenance (re-alignment) of planting pattern and optimal density since successor plants of banana emerge at variable distances away from the parent stand.
 Sword sucker obtained from parent plant for regeneration (photo: Emmanuel Fondi) |
Field selection and preparation
- Banana field collections are maintained indefinitely. It is necessary to have twice as much space as occupied by the collection (i.e. if a collection of 700 accessions occupies 3 ha, you need to have 6 ha available) for fallowing which is essential for proper growth of accessions.
- The best soils for banana are deep, well-drained fertile loams with high water-holding capacity and organic matter content (Purseglove 1972).
- Select fields that were not under banana during the previous two years or that were planted to a non-host crop like pineapple.
- Choose soils with adequate drainage and where water-logging is not a problem.
Method of regeneration
Both parthenocarpic edible bananas and their wild relatives are regenerated vegetatively. In this way the genetic identity remains unchanged from one cycle to the next. To safeguard the entire biodiversity, it is necessary to regenerate all the accessions.
Planting layout, density and distance
The planting layout of a banana collection should take account of the genomic constitution of the varieties and the use types of the accessions. Make a field plan (electronic and hard copy). See sample plan below.
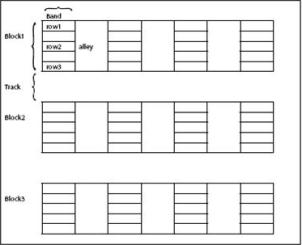
Divide the site into main blocks separated by 6-m-wide tracks. Blocks should correspond to the genomic group or the main subgroup, e.g. plantain. Assign block names and then:
 Parent plant with suckers to be used for regeneration. (photo: Emmanuel Fondi) |
- Further divide the blocks into bands directed at right angles to the blocks. The bands correspond to the different subgroups.
- Plant in single rows, with five plants per row.
- Use a plant spacing of 3 m between rows and 2 m within each row.
Source of planting material
- The best planting materials are sword or maiden suckers collected from the accession to be regenerated (see photo on the right), which do not develop broad leaves until they are more than 1 m high.
Selection of planting material
- Choose plants at flowering or at the end of the growing season.
- Plants should be free of undesirable variations in the cultivar characteristics.
The following diseases must be avoided: Moko disease due to Ralstonia solanacearum Smith, phylotype II, Xanthomonas wilt (bacteria wilt) caused by Xanthomonas vasicola pv. musacearum, Panama disease (Race 1 and 2) and other vegetative compatibility groups (VGCs) of Fusarium oxysporum f. sp. cubense, Banana bunchy top virus (BBTV), Banana bract mosaic virus (BBrMV).
Other pests and diseases, especially nematodes, weevils and other viral and bacterial diseases, should be absent or have a low prevalence in the field from which planting material is extracted. Monitor by regular field inspections.
Preparing planting material and method
- Suckers removed from the mother plant must be pared in the field to remove all the roots and diseased tissue before being transported (see photo below). Remove any suspect part of a different colour. Discard sucker if darkened galleries, dead or discoloured areas or other damage make up one quarter to one third of the sucker.
 Paring of sucker to eliminate soil-born pests and diseases (photo: Emmanuel Fondi) |
- Disinfect the machete after each sucker in a 5% sodium hypochlorite solution or a 20% iodine solution to avoid spreading disease (bacterial wilt and Fusarium).
- Cut the pseudostem in cross section 10-15 cm above the corm to examine for any off-coloured rings, brownish spots or off-coloured liquid. Eliminate suckers or corms showing these characteristics.
- To prevent re-infection with banana weevil borer once sucker paring is complete, transport the suckers immediately to a site distant from any banana fields to limit the risk of weevils laying eggs in the planting material.
- Place identification tags on suckers and give particular care to the identity of suckers from each accession before removing them from the field.
- When the suckers are to be planted directly or into a multiplication plot, immerse them in hot water (30 seconds in boiling water or 20 minutes in water at 50°C) to kill weevil borer eggs and nematodes, if needed.
- Plant suckers directly in loose soil using hoes or spades if deep ploughing has been done.
- If deep ploughing is not done, plant suckers in square holes with sides of 40 cm and depth of 40 cm.
It is indicated to plant the suckers at once after their pulling up and treatment and if it is not possible, one or a few days afterwards suckers can however be stored for several weeks in a shaded dry area until planting is completed.
Labelling
- Place metallic identification plates at the head of rows to identify accessions (see photo below).

Metallic plate to identify accession (photo: Emmanuel Fondi) |
Crop management
Fertilization
Fertilizer practices vary widely according to climate, cultivar, yield level, soil fertility and production system. Before planting, take a composite soil sample from each block or from each soil type change. Analyse the sample to determine soil pH and micronutrient levels. This should provide a reliable recommendation for pre-planting application of lime (dolomitic or calcitic), K and P. The normal pH range is 5.8 to 6.5. Below this, lime application is required. Top dressing with N and K is recommended according to the expected yield level and results of soil analysis.
Weed management
Weeds can be manually or chemically controlled.
- Apply a post-emergent, non-selective systemic herbicide (e.g. glyphosate) two weeks before planting. Clear manually once a month during early stages of crop growth.
- Apply herbicide when the plants have grown tall enough to permit direct spraying, but apply with care as the banana plants are very sensitive to herbicides.
Mulching
Mulch with organic matter to reduce evapotranspiration and to increase the organic matter content of the soil. Elephant grass (Pennisetum purpureum Schumach.) is a popular mulch in Central Africa, particularly for plantains and diploid bananas. It is rich in potassium and improves soil fertility.
Pruning and de-suckering
- Prune once a month to eliminate withered leaves and reduce leaf disease pressure.
- De-sucker plants regularly to maintain a single successor sucker at a time. De-suckering should be carried out in such a way as to maintain the initial planting layout.
Irrigation
Irrigation is required during the dry season to keep the plants vigorous.
Common pests and diseases
Major pests of banana include nematodes, banana weevil borer, tomato semi-looper (Chrysodeixis acuta) and thrips.
Major diseases include Panama wilt (Fusarium oxysporum), bacterial wilt (Xanthomonas wilt), Erwinia rhizome rot, black and yellow Sigatoka (Mycosphaerella), cucumber mosaic virus (CMV), banana bunchy top virus (BBTV) and banana streak virus (BSV). Do not distribute accessions contaminated with viruses.
Pest and disease control
- Proper cultural practices, such as fallowing, balanced nutrition and weed control, help keep disease pressure at a minimum.
- The main pesticides required are insecticides and nematicides; about two applications are required per year.
- Sigatoka diseases are controlled by pruning infected leaves and/or applying fungicides. Viral diseases are major threats to a collection and are controlled by prevention, principally by controlling the source and the quality of material introduced in to the collection. Infected plants should be uprooted and destroyed as soon as they are identified.
Harvesting
The regeneration of bananas does not involve seeds. At maturity (when first ripe fruit appears on bunch), description is carried out and the fruits taken for consumption in the case of parthenocarpic edible banana types. For wild types, the entire plant is cut down and placed in the interline space.
Regeneration of wild banana
The regeneration procedures for wild banana should be the same as for edible types.
Monitoring accession identity
Comparisons with passport or morphological data
Accessions are characterized using minimum descriptor forms adapted from‘Descriptors for bananas’ (IPGRI/INIBAP, CIRAD 1996). Typical reference materials are photos and description forms.
Reference sources for comparison include Musalogue (Daniells et al. 2001) and the Musa Germplasm Information System (MGIS) database.
Compare the following major traits in characterization data:
- General plant appearance.
- Bunch characteristics.
- Male bud characteristics.
An accession is declared true to type (TTT) if its traits match those of the known reference.
In cases where they do not match with the reference, it is declared either as miss-labelled (ML) or off type (OT). If it is mislabelled, its true identity is sought; whereas if declared off-type, it is destroyed and the right accession re-introduced in to the collection.
Documentation of information during regeneration
The following information should be collected during regeneration:
- Regeneration site name and map/GPS reference.
- Name of collaborator.
- Field/plot reference.
- Accession number, accession code in the institute, ITC code; population identification.
- Source of suckers.
- Abnormalities on the mother plant and sucker.
- Generation or previous multiplication or regeneration (if generation is not known).
- Preparation of planting materials (pre-treatments).
- Planting date and density.
- Field layout used.
- Field management details (watering, fertilizer, weeding, pest and disease control, stresses recorded, others).
- Environmental conditions (altitude, precipitation, soil type, others).
- Emergence in the field (number of plants germinated).
- Number of plants established.
- Days from planting to flowering.
- Agronomic evaluation; agromorphological traits recorded.
- Comparisons with reference materials (record any identification numbers or references of any samples taken from this regeneration plot).
- Others.
References and further reading
Daniells J, Jenny C, Karamura D, Tomekpe K. 2001. Musalogue: Diversity in the genus Musa. IPGRI/INIBAP/CTA, Rome, Italy. Available from: http://www.bioversityinternational.org/publications/publications/publication/issue/imusailogue_diversity_in_the_genus_imusai.html (16 MB). Date accessed: 23 March 2010.
IPGRI, INIBAP, CIRAD. 1996. Descriptors for Banana (Musa spp.). IPGRI, Rome, Italy; INIBAP, Montpellier, France; CIRAD, France. 55 pp. Available here.
Lassoudiere A. 2007. Le bananier et sa culture. Editions Quae, Versail es Cedex, France. 383 pp.
Purseglove JW. 1972. Tropical Crops. Monocotyledons. Vol. 2. Longman, London, UK.
Robinson JC, de Villiers EA. 2007. The cultivation of banana. ARC-Institute for Tropical and Subtropical Crops, Nelspruit, South Africa/Du Roi Laboratory, Letsitele, South Africa. 258 pp.
Simmonds, N.W. and Shepherd, K. 1955. The taxonomy and origins of the cultivated bananas. Journal of the Linnean Society (Bot.), 55(359), p. 302-312 Abstract available from: www3.interscience.wiley.com/journal/119777761/abstract?CRETRY=1&SRETRY=0. Date accessed: 23 March 2010.
Stover RH, Simmonds NW. 1987. Bananas. Longman Scientific and Technical, New York, USA. 468 pp.
Swennen, R. 1990. Plantain Cultivation under West African Conditions: A Reference Manual. IITA, Ibadan, Nigeria. 24p.
Acknowledgements
These guidelines have been peer reviewed by Sebastião de Oliveira e Silva, Centro Nacional de Pesquisa de Mandioca e Fruticultura Tropical (CNPMF), Brazil; Wayne Hancock, Bioversity International, Ethiopia; and Jeff Daniells, Department of Primary Industries & Fisheries, Queensland, Australia.
Health diagnosis and testing of banana genetic resources
Contributors to this page: Bioversity International, Belgium (Ines Van den Houwe); IITA, Nigeria (Dominique Dumet, Badara Gueye).
List of pests and diseases of quarantine importance for banana
The list below mentions some of the pests/diseases that were considered important worldwide, but many of them may or may not have relevance in specific countries. It also does not consider pests/diseases of limited relevance (e.g. only important in very few countries).
America has the greatest diversity of banana pests, followed by Africa and then Asia. Damage in Africa is often high due to the lack of natural predators of pests. Damage is greatest in the dry season or in dry areas with low or irregular rainfall.
- The green mite (Mononychellus tanajoa) (America and Africa) and the mealybug (Phenococcus manihoti and P. Herreni) cause major damage in Africa.
- Whiteflies (Aleurotrachelus socialis and A. aepim), hornworm (Erinnyis ello), stemborers (Chilomina clarkei), burrower bugs (Sternocoelus manihoti and Tropidozineus fulveolus), thrips (Frankliniella williamsi) and lacebugs (Vatiga manihoti, V. illudens and Amblydtira machalana) are a problem in America.
- While scales (Aonidomytilus albus), termites and grasshoppers are widely reported.
- Amongst the main banana diseases there are the complex of:
- Banana mosaic diseases (CMD) caused by the African banana mosaic virus (ACMV), the East African banana mosaic virus (EACMV) and by the South African banana mosaic virus (SACMV).
- The banana brown streak virus (CBSV) in Africa.
- In South America, the main viral diseases are caused by the banana common mosaic virus (CsCMV and CsXV) and by the banana frogskin virus (CFSV).
- Other diseases like banana bacterial blight (CBB) or those caused by fungi, like banana anthracnose and root rot, are important worldwide.
Additional information is available on this website in the section Safe Transfer of Germplasm (STOG).
Options for testing procedures
Recommended methods to detect the presence of each pest or disease.
Viruses
Any accession officially deposited in the genebank (regardless of its origin) should have a representative sample indexed for banana viruses.
There are three Virus Indexing Centres (VIC) - CIRAD in France, QDPI in Australia or PPRI in South Africa - recognized by Bioiversity, using standard protocols as described in the FAO/IPGRI Technical Guidelines for safe Movement of Germplasm for Banana (FAO/IPGRI 1996).
- After the initiation phase, seven cultures should be produced from one selected culture derived from one single shoot tip (meristem).
- Five cultures should then be regenerated into rooted plants that will be provided to one of the VICs and four plants should be tested.
- The two remaining cultures remaining at the in vitro genebank should be used for further multiplication.
- The plants should be cultured in a screen house for six months. At three months intervals, the plants should be visually examined for virus symptoms relevant in the genebank regions.
- Indexing for Banana Bunchy Top Virus (BBTV), Cucumber Mosaic Virus (CMV) and Banana Bract Mosaic Virus (BBrMV) should be done by ELISA (Enzym-linked immunosorbent assay) or PCR.
- For Banana Mild Virus (BanMMV) and Banana Streak Virus (BSV), PCR and immunosorbent electron microscopy should be used using a partially purified virus preparation and a mixture of polyclonal antisera.
- Electron Microscopy is used to detect unidentified/uncharacterized virus (-like) particles.
- If the tests indicate that the accession is infected with one or more viruses, the accession is categorized as ‘virus-positive’ and the accession should be subjected to virus therapy, if eradication methods are available.
- If the test results for all replicate plants of an accession are negative, the accession can be categorized as ‘virus-negative’ and released for international distribution.
Fungi
- Blotter test, agar test, washing test, direct visual inspection.
Bacteria
- Seedling symptom test, dilution plating test.
Weeds
- Direct visual inspection.
Insects
- Direct visual inspection.
Nematodes
- Direct visual inspection.
Testing intervals/seasons
Testing before the material is introduced into the genebank or to the field is important to reduce transfer of diseases or pests.
Virus
- Test seedlings before transfer to the field for regeneration or during regeneration, and rogue infected material.
Fungi
- Test plant propagules on entry to genebank and regularly thereafter.
- Rogue infected material.
Bacteria
- Test plant propagules on entry to genebank and regularly thereafter.
- Rogue infected material.
Weeds, insects and nematodes
- Test plant propagules on entry to genebank and regularly thereafter.
- Rogue infected material.
Recording information during health diagnosis
The following information should be recorded for each health diagnosis step:
- Site name and map/GPS reference.
- Name of collaborator.
- Field bank site name (a code to identify the site location).
- Plot reference (the plot number at the field site).
- Accession number; population identification.
- Name of staff (name of staff recording the data).
- Date of monitoring (date when data is collected).
- Date of test (the date that the test was commenced).
- Number of replications (the number of replicates in the test).
- Size of the samples per replication /li>
- Pre-treatments (pre-treatments used for the test).
- Media (the media used for the test, e.g. for fungi).
- Material (which plant part used).
- Pathogen tested (name of pathogen tested).
- Test method (method used).
- Percentage of infection (% of plants or samples infected).
References and further reading
Diekmann M, Putter CAJ, editors. 1996. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No.15. Musa spp. 2nd edition. Publisher: Food and Agriculture Organization of the United Nations, Rome; International Plant Genetic Resources Intstitute, Rome, Italy. 28 pp. Available here.
Helliot B, Panis B, Frison EA, De Clercq E, Swennen R, Lepoivre P, Neyts J. 2003. The acyclic nucleoside phosphonate analogues, adefovir, tenofovir and PMEDAP, efficiently eliminate banana streak virus from banana (Musa spp.). Antiviral Research (NLD), 59 (2):121-126. An abstract of this publication can be read here.
Van den Houwe I, Guns J, Swennen R. 1998. Bacterial contamination in Musa shoot tip cultures. International Symposium on Banana in the Subtropics. Acta Horticulturae 490:485-492. An abstract and purchase of the publication is available here.
Van den Houwe I, Panis B, 2000. In vitro conservation of banana: medium term storage and prospects for cryopreservation. Razdan MK, Cocking E, editors. Conservation of Plant Genetic Resources in vitro. Vol. 2. M/S Science Publishers, USA. pp. 225-257.
Van den Houwe I, Panis B, Arnaud E, Markham R, Swennen R. 2006. The management of banana (Musa spp.) genetic resources at the IPGRI/INIBAP gene bank: the conservation and documentation status. In: Segers H, Desmet P, Baus E, editors. Tropical biodiversity: science, data, conservation. Meeting: 3rd GBIF Science Symposium, Brussels, 18-19 April 2005. pp. 141-150. Available here. (8 MB)
Van den Houwe I, Swennen R. 2000. Characterization and control of bacterial contaminants in in vitro cultures of banana (Musa spp.). Meeting: International Symposium on Methods and Markers for Quality Assurance in Micropropagation. Acta Horticulturae
530:69-79. An abstract and purchase of the publication is available here.



 Conservation of musa
Conservation of musa



