Cryopreservation of banana apical meristems
.jpg) View the full publication on Cryopreservation of Musa germplasm by clicking on the icon above (1.35 MB) |
Contributors to this page: Bioversity International, Belgium (Bart Panis).
The content of this page was extracted from Panis B. 2009. Cryopreservation of Musa germplasm: 2nd edition. Technical guidelines No. 9 (F. Engelmann and E. Benson, eds). Bioversity International, Montpellier, France.
Cryopreservation of in vitro grown shoot tips of banana through vitrification was originally reported by Thinh and co-workers (Thinh et al. 1999). Using this method, regeneration percentages were often low and unpredictable. Therefore, the technique was further improved and adapted at KULeuven to make it applicable to a wide variety of cultivars (Panis et al. 2005).
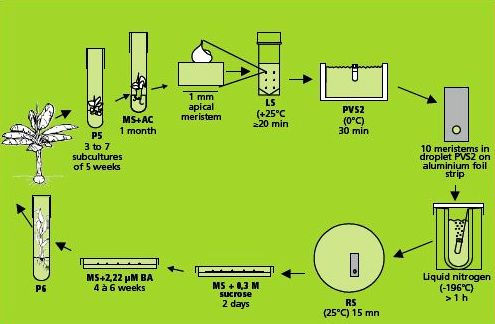
This method is illustrated in the figure below.
|
Cryopreservation of individual meristems (photo: KULeuven/Bioversity) |
Preparation of plant materials
|
Production of robust, rooted in vitro plants of banana cv. ‘Williams’ (photo: KULeuven/Bioversity) |
Preparation of in vitro plantlets
All accessions were obtained from the Bioversity Musa germplasm in vitro collection (KULeuven, Belgium). This collection contains edible banana cultivars, as well as wild relatives.
- Shoot cultures are grown in 25x150 ml test tubes on 25 ml p5 medium. P5 medium contains semi-solid Murashige and Skoog (MS) medium supplemented with 30 g/L sucrose, 10 μM BA and 1 μM IAA and solidified with 2 g/L gelrite (Banerjee and de Langhe 1985). They are cultured at 25±2°C under continuous 50 μE m-2 s-1 illumination provided by 36W Osram cool-white fluorescent tubes. The pH is adjusted to 5.8 prior to autoclaving.
- From these multiplying cultures, shoots of 3-5 cm in length are separated and transferred to rooting medium in test tubes. The rooting medium has the same composition as p5, but it is devoid of plant growth regulators and supplemented with 0.5 g/L active charcoal.
- After one month, robust and well rooted in vitro plants are obtained with a corm diameter of 5-8 mm that forms an appropriate source for the excision of apical meristems (see photo).
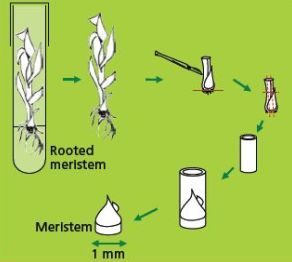
Dissection and selection of apical meristems
Like many other monocots, banana apical meristems are tightly covered with several layers of whitish, tubular, immature leaves.
- Individual apical meristems are excised under a binocular microscope. Leaves are removed one by one until the apical dome is visible, but still partially covered by 1-2 young leaf primordia (see figure and photo below).
- The leaf base (corm tissue) is 1 mm in diameter. In order to excise corm pieces of exactly this size, a millimetre grid graph paper is placed underneath the transparent sterile plastic Petri dish in which the meristems are excised.
- The dissected meristems are transferred into the loading solution (in the dark at room temperature). Tips that are slightly damaged or are not in the correct stage (i.e. the meristem is too much or too little covered by leaf primordia) are excluded from cryopreservation.
A skillful and trained technician can isolate a maximum of ten meristems an hour (roughly, six minutes/isolated meristem).
|
Illustration of meristem isolation. Leaves are removed one by one until the apical dome is visible but still partially covered by 1-2 young leaf primordia |
Partly-covered apical meristems of banana cv. ‘Williams’ (photo: KULeuven/Bioversity)
|
Cryopreservation through droplet vitrification
Loading, dehydration and rapid freezing
|
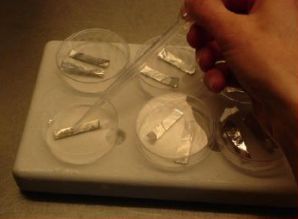
Transfer of meristems to a droplet of PVS2 solution (of about 15 μl) on a strip of aluminium foil (5x20 mm) with a 2 ml plastic Pasteur pipette (photo: KULeuven/Bioversity) |
- The filter sterilised loading solution contains 2 M glycerol and 0.4 M (= 136.8 g/L) sucrose dissolved in MS medium (pH 5.8). The excised meristems are left in the loading solution in a 20 ml plastic vessel until all of them are dissected. The exposure time thus varies between 20 min and 5 hrs. Previous research has shown that regrowth of banana meristems is not influenced by loading solution exposure time (Panis et al. 2005). Although the precise mechanism of loading is not yet fully understood, it has been proven for different plant species that loading can dramatically enhance the tolerance of isolated meristems to dehydration by the vitrification solution (Matsumoto et al. 1994, Takagi et al. 1997).
- After loading, the solution is replaced by ice-cooled PVS2 solution. The PVS2 solution consists of 30% (w/v) (3.26 M) glycerol, 15% (w/v) (2.42 M) ethylene glycol (EG), 15% (w/v) (1.9 M) DMSO and 0.4 M (= 136.8 g/L) sucrose (Sakai et al. 1990).
- All these compounds are dissolved in MS medium, pH adjusted to 5.8, followed by filter sterilization. The meristems are subjected to the PVS2 solution for 30-40 min. at 0°C.
- Five min. before the end of the treatment, ten meristems are transferred individually to one droplet of PVS2 solution (of about 15 μl) on a strip of aluminium foil (5x20 mm) with a 2 ml plastic Pasteur pipette (see photo).
- To keep the temperature of the strip around 0°C during the manipulations, the aluminium strip is placed in a plastic Petri dish placed on top of a frozen cooling element. After the PVS2 treatment, the aluminium strip is plunged into liquid nitrogen with a fine forceps.
- For permanent cryostorage, the frozen foil is quickly transferred to a 2 ml cryotube filled with liquid nitrogen and closed.
Storage, re-warming and unloading
- The meristems are kept in liquid nitrogen for at least 20 min. For re-warming, aluminium foil strips are rinsed in 10 ml unloading solution in a small Petri dish at room temperature. After a few seconds, meristems are released from the strip of aluminium foil and kept for another 15 min in the unloading solution. As such, the toxic PVS2 solution is removed whilst re-warming and replaced by a less-toxic unloading solution. The unloading solution consists of 1.2 M (= 410.4 g/L) sucrose dissolved in MS medium (pH 5.8).
Recovery
- Unloaded meristems are placed onto two sterile filter papers on top of semi-solid hormone-free MS medium containing 0.3 M (=102.6 g/L) sucrose.
- After two days, the meristems are transferred onto regeneration medium without filter papers. The first week of culture always takes place in the dark.
Four to six weeks following cryopreservation, four types of reactions can be distinguished:
(i) white shoot tips resulting from an immediate death of the tissue without blackening.
(ii) completely or partially black shoot tips, indicating that there was enzymatic reaction following cryopreservation (production and oxidation of polyphenols).
(iii) unorganized callus growth, representing the outgrowth of small isolated areas of the apical dome and/or primordial tissues.
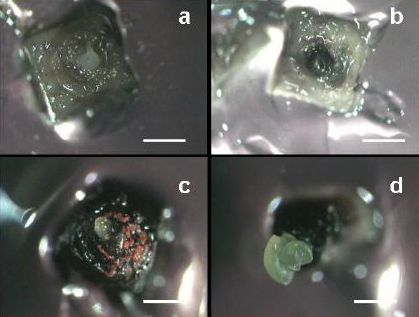
(iv) shoot tip regeneration resulting from the survival of a substantial part of the apical dome (see figures a-d).
 |
|
|
Reaction of apical meristems towards cryopreservation 30 days after re-warming (a) No growth; the meristem remains white; (b) Blackening without further growth; apical dome reacts by formation of polyphenolic compounds that oxidize; (c) Callus formation; watery, non-morphogenic callus; and (d) Shoot regeneration (bar = 600 μm)(photo: KULeuven/Bioversity) |
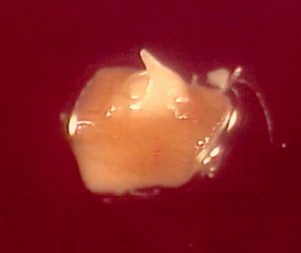
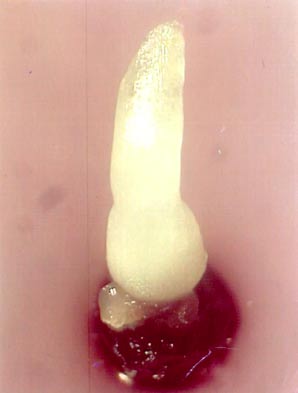
One month after re-warming, a 0.5 cm long shoot can be observed (see photo). Calluses never produced shoots.
|
Recovered shoots from cryopreserved apical meristems of banana cv. ’Williams’ 1 month after re-warming |
Discussion and perspectives
Different Musa genotypes have been cryopreserved using this protocol (Thinh et al. 1999, Panis et al. 2005). Post re-warming regeneration percentages range between 20% and 85% (with an average of 53%). These recovery percentages are relatively genotype independent. It was, however, observed that genotypes with more B-genome survive significantly better than those with solely the A-genome (Panis et al. 2005). Microscopic observation of the recovery of cryopreserved meristems (Helliot et al. 2003) has revealed that:
(i) the whole dome of the isolated meristem withstands exposure to liquid nitrogen.
(ii) no regenerable post cryopreservation callus is formed. Therefore, it would appear that somaclonal variation is unlikely to occur.
The main limitations to this procedure are as follows:
- Post cryopreservation viability/regeneration percentages vary depending on the operator. Considerable experience in the dissection of tiny and fragile banana apical meristems is required before this cryopreservation protocol can be applied (using this method only 60 meristems can be excised and cryopreserved in one day).
- Low regrowth may be due to low quality meristems (tips that are slightly damaged or are not at the correct stage or too much or too little covered by leaf primordia). The quality of the donor plants (more light, fewer plantlets in containers) should be carefully monitored.
- Using the droplet vitrification method, some researchers have shown some concern with respect to the effects of the direct contact of liquid nitrogen with the plant material. Supposed problems like contamination and loss of material were, however, never encountered.
This droplet vitrification protocol was recently successfully applied to a wide range of plant species such as potato, ulluco, sweet potato, chicory, strawberry, taro, Pelargonium L., date palm, thyme, olive, Byrsonima, snowdrop, apple and hop and might thus be considered as the first generally applicable protocol (Panta et al. 2006, Gallard et al. 2008, Sant et al. 2008, Feki et al. 2011, Sánchez-Romero and Panis 2009, Marco-Medina et al. 2010, Condello et al. 2011, Maslanka et al. 2013, Coutinho Silva et al. 2013).
References and further reading
Banerjee N, De Langhe E. 1985. A tissue culture technique for rapid clonal propagation and storage under minimal growth conditions of Musa (banana and plantain). Plant Cell Reports 4:351-154.
Condello E, Caboni E, André E, Piette B, Druart P, Swennen R, Panis B. 2011. Cryopreservation of apple in vitro axillary buds using droplet-vitrification. CryoLetters 32:175-185.
Coutinho Silva L, Paiva R, Swennen R, André E, Panis B. 2013. Shoot-tips cryopreservation by droplet vitrification of Byrsonima intermedia A. juss.: a wooden tropical and medicinal plant species from Brazilian Cerrado. CryoLetters 34: 338-348.
Feki L, Bouaziz N, Sahnoun N, Swennen R, Drira N, Panis B. 2011. Palm cryobanking. CryoLetters 32:451-462.
Gallard A, Panis B, Dorion N, Swennen R, Grapin A. 2008. Cryopreservation of Pelargnonium apices by droplet-vitrifications. CryoLetters 29 (3):243-251.
Helliot B, Swennen R, PouMay Y, Frison E, Lepoivre P, Panis B. 2003. Ultrastructural changes associated with cryopreservation of banana (Musa spp.) highly proliferating meristems. Plant Cell Reports 21:690-698.
Marco-Medina A, Casas JL, Swennen R, Panis B. 2010. Cryopreservation of Thymus moroderi by droplet vitrification. CryoLetters.
31 (1):14-23.
Maslanka M, Panis B, Bach A. 2013. Cryopreservation of Galanthus elwesii Hook. apical meristems by droplet vitrification. Cryo Letters, 34: 1-9.
Matsumoto T, Sakai A, Yamada K. 1994. Cryopreservation of in vitro-grown apical meristems of wasabi (Wasabi japonica) by vitrification and subsequent high plant regeneration. Plant Cell Reports 13:442-446.
Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco cell cultures. Physiologia Plantarum 15:473–497.
Panis B. 2009. Cryopreservation of Musa germplasm: 2nd edition. Technical Guidelines No. 9 (F. Engelmann and E. Benson, eds). Bioversity International, Montpellier, France. Available here.
Panis B, Piette B, Swennen R. 2005. Droplet vitrification of apical meristems: a cryopreservation protocol applicable to all Musaceae. Plant Science 168:45-55.
Panta A, Panis B, Ynouye C, Criel B, Swennen R, Roca W. 2006. Improvement of potato cryopreservation for the long-term conservation of Andean landraces at CIP. 43rd Meeting of the Society for Cryobiology in association with the Society for Low Temperature Biology. Hamburg, Germany, 24-27 July 2006.
Sakai A, Kobayashi S, Oiyama I. 1990. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Reports 9:30-33.
Sánchez-Romero C, Swennen R, Panis B. 2009. Cryopreservation of olive embryogenic cultures. CryoLetters 30 (5):359-372.
Sant R, Panis B, Taylor M, Tyagi A. 2008. Cryopreservation of shoot-tips by droplet vitrification applicable to all taro (Colocasia exculenta var. esculenta) accessions. Plant Cell Tissue and Organ Culture 92:107-111.
Takagi H, Thinh NT, Islam OM and Senboku T. 1997, Cryopreservation of in vitro-grown shoot tips of taro (Colocasia esculenta (L.) Schott) by vitrification. 1. Investigation of basic conditions of the vitrification procedure. Plant Cell Reports 16:594-599.
Thinh NT, Takagi H, Yashima S. 1999. Cryopreservation of in vitro-grown shoot tips of banana (Musa spp.) by vitrification method. CryoLetters 20 (3):163-174.
Comments
- No comments found






Leave your comments
Post comment as a guest