Distribution of cassava genetic resources
Contributors to this page: CIAT, Colombia (Daniel Debouck); IITA, Nigeria (Dominique Dumet); Bioversity International/ILRI, Ethiopia (Alexandra Jorge); INIA, Peru (Llerme Rios); independent consultant (Clair Hershey).
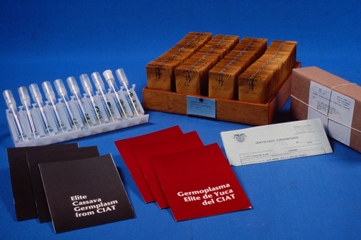
An important role of in vitro genebanks is the distribution of germplasm to different users.
Click here to open a PDF document that shows how to disseminate germplasm in detailed steps.
|
Contents: |
 Cassava dissemination (photo:IITA) |
Common policies on distribution and access to plant material
- Follow the International Treaty on Plant Genetic Resources for Food and Agriculture (ITPGRFA) (www.planttreaty.org) for in-trust germplasm using the Standard Material Transfer Agreement (SMTA).
Policy exceptions
- None.
National laws and regulations
It is essential to follow the terms and conditions in the host country agreements:
- Export permits.
- Phytosanitary certificate.
- Certificate of origin.
International laws and regulations
The shipment of plant material should be sent with the SMTA under the International Treaty for Plant Genetic Resources for Food and Agriculture.
Phytosanitary regulations
- Phytosanitary certificates are needed for most countries.
- See the safe transfer of germplasm (STOG) section in this website (this is essential to avoid the spread of pests and diseases).
User related issues for distribution
Feedback to users
Factors that can influence the delivery of plant material:
- Respond to requests with lists of material, forms and conditions of access and SMTA as soon as possible after receipt of request (users may not know about the conditions so it is better to inform them before proceeding with the request).
- Provide passport and germination data with requests.
Recommended procedures that ensure the material distributed matches the client request:
- On specific requests, match accession numbers with specified request.
-
If accession numbers are not specified, match accessions to users needs. These could include:
- Species.
- Plant habit to fit the crop system.
- Environment.
- Specific traits required, such as disease resistance.
- Use (e.g. consumer-related traits).
Feedback from users
The most relevant information required to be received from users:
- Information on characterization and evaluation/use (information on performance in one area allows better selection of germplasm for similar areas).
Quantity of material recommended to be distributed
Cassava accessions typically consist of a single clone, i.e there is no genetic variation within an accession. A single plant can represent the full diversity of the accession. The main consideration on quantity of material to send is to assure that the recipient has a very high probability to successfully recover the accession from in vitro culture.
- Three to five seedlings per accession are normally adequate.
Check availability
Availability in stock
- Check availability of vegetative material in the field or laboratory.
- Distribution of the requested material should not cause the accession to fall below the minimum stock or distribution of vegetative cuttings cause plant death.
- Accessions with little vegetative material or those which are not healthy should not be distributed.
Checking passport data
- Passport and evaluation data can be checked to ensure that the accession meets the requestor’s needs (this avoids waste of germplasm by sending material that the requestor does not need or want).
Preparing vegetative material for distribution
- Register the request: track requests according to the dates they are received (this allows requests to be handled on a first come first served basis).
- Prepare a list of accessions available: generate lists of accessions that meet users' needs.
- Check requirements for Standard Material Transfer Agreements (SMTA).
- Micropropagate or multiply the necessary plant material.
- Transfer material into multiplication or rooting media to promote the aerial growth and roots, to facilitate acclimatization.
- Prepare shipment, after 4-6 weeks of growth.
- Check the vigour of the plants before packaging. Only seedlings showing well-developed root and shoot systems should be considered for distribution.
- Pack the tubes carefully in boxes, with all the necessary labels. Use labels with good adhesive and clear printing (this avoids errors and mixing during shipping).
- Acquire the Phytosanitary Certificate to include in the shipment.
- Acquire the Import Permit.
-
Elaborate list of information to include in the shipment:
- Accession number.
- Accession identification.
- Crop name.
- Taxonomic identification.
- Country of origin.
- Biological status.
- Collecting location.
- Source.
- Characterization data used to verify accessions should be provided upon request.
- Cover letter, reminding users of the terms and conditions of access and requesting feedback (important to make contact with the user for future feedback).
- Include a handbook of procedures, explaining how the material should be handled. In vitro seedlings need special attention prior to transferring to field conditions.
- Register information into the plant genetic resources database.
|
|
Dispatching the plant material
- Pack the plant material for secure shipment,and to avoid damage or mixing the material.
- Attach documents e.g. the seed list, SMTA, Phytosanitary Certificate, Import Permit, GMO-free certificate in a strong envelope or a cardboard box.
- Attach a copy of the Phytosanitary Certificate, Import Permit and list of material to the outside of the box, if the material is being dispatched to another country.
- A copy of the SMTA must be attached to the outside of the envelope or box. Use of the material constitutes agreement with the terms of the SMTA.
- Label the envelope/box with the complete mailing address of the requester.
- Include a reply form (a reply form should be used to acknowledge that the material has been received by requester in good condition).
- Use courier or other rapid means of transit (method should avoid heating and delays in transport).
- Record date and method of shipping (shipping details are important for tracking during shipping).
- Update the genebank inventory.
Recording information during distribution
System for tracking material/inventory system for distribution
- Update related data tables in the database management system (a database system allows easy and fast access to data and allows macros to be written for routine operations).
- Use labels with good adhesive and clear printing (this avoids errors and mixing during shipping).
References and further reading
Ceballos H. 2006. Cassava research at CIAT [poster]. Centro Internacional de Agricultura Tropical (CIAT), Cali, Colombia. Available from: http://webapp.ciat.cgiar.org/news/pdf/poster02_scmeeting_06.pdf. Date accessed: 26 August 2010.
Guevara CL, Mafla G. 1996. Manihot collections held at CIAT. In: Engelmann F, editor. Management of field and in vitro Germplasm collections. CIAT, Cali, Colombia. pp. 109-112.
Mafla G, Roa JC, Debouck DG. 2004. Observations about the distribution of cassava germplasm from an international collection. Poster presented at the CBN-V. Available from: http://isa.ciat.cgiar.org/urg/urgweb_folder/files/posters/CBN-V.%20G%20Mafla%20ppt.pdf. Date accessed: 30 August 2010.
Mafla G, Roa JC, Flor NC, Aranzales E, Debouck DG. 2006. Distribution of cassava germplasm from an international genebank: a service to the global agriculture. Poster presented at the first meeting of the Governing Body, ITPGRFA, Madrid, Spain, 12-16 June 2006. Available from: http://ciat-library.ciat.cgiar.org/Articulos_Ciat/CIAT40years.pdf. Date accessed: 30 August 2010.
Comments
- No comments found






Leave your comments
Post comment as a guest