Cryopreservation of banana meristem clusters (cauliflower-like structures)
.jpg) View the full publication on Cryopreservation of Musa germplasm by clicking on the icon above (1.35 MB) |
Contributors to this page: Bioversity International, Belgium (Bart Panis).
The content of this page was extracted from Panis B. 2009. Cryopreservation of Musa germplasm: 2nd edition. Technical guidelines No.9 (F. Engelmann and E. Benson, eds). Bioversity International, Montpellier, France.
A second type of regenerative meristematic tissue in banana which has been successfully cryopreserved is the highly proliferating (sometimes called cauliflower-like) meristem clusters. This tissue type was originally produced as a starting material to initiate embryogenic cell suspension cultures in bananas (Dhed’a et al. 1991, Strosse et al. 2006, Schoofs 1997).
Two cryopreservation techniques applied to highly proliferating ‘cauliflower-like’ meristem clusters are described below:
Contents: |
Preparation of plant materials
|
Meristem cultures of banana cultivars Nakitengwa (AAA highland banana), Williams (AAA group) and Bluggoe (ABB group) on (left) p5 medium (containing 10μM BA), and (right) p4 medium (containing 100μM BA)(bar = 2 cm) (photo: KULeuven/Bioversity) |
Production of ‘cauliflower-like’ meristem clusters
To produce this kind of material in all Musa accessions, meristem cultures are transferred to a medium containing a high concentration of BA (P4 medium, see Appendix 1). Every one to two months, the material is subcultured and small clumps of ‘cauliflower-like’ meristems are selected and transferred to fresh medium (Strosse et al. 2006). The high BA concentration (up to 100 μM) in the P4 medium suppresses outgrowth of meristems, thereby favouring the formation of numerous white apical domes (see photo on the right). Repeated subculturing is necessary and can take
4-12 months.
Preculture of meristem clumps
- Following the appearance of ‘cauliflower-like’ clusters, and four weeks after the last subculture, white meristematic clumps of about 4mm diameter, each containing at least four apical domes, are excised and transferred onto preculture medium (P5 + 0.4 M (= 136.8 g/L) sucrose) for four weeks. They are cultured at 25°C ± 2°C in darkness.
Discussion and perspectives
The quality of ‘cauliflower-like’ meristem clumps may be too poor for use in cryopreservation experiments (meristematic tissue versus corm tissue is too low and/or the explant shows too much blackening). This can be due to the fact that the cultivar belongs to a ‘difficult’ genomic group (for example East African highland bananas and many plantains). Previously, proliferation was only obtained by using BA at extremely high, nearly toxic, concentrations (100 μM). Prolonged culture on 100 μM BA containing media, therefore, often results in a quality decrease (loss of the typical ‘cauliflower-like’ characteristics) of the cultures. Recently, the use of alternative cytokinins like thidiazuron (TDZ) at lower concentration (1 μM) proved to increase proliferation rates (Strosse et al. 2008).
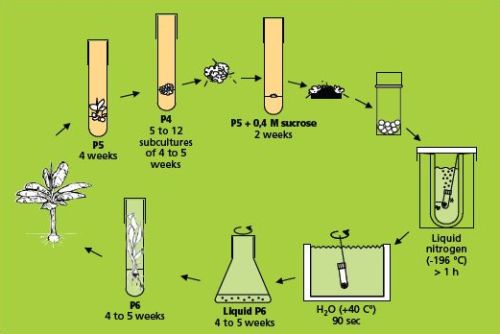
The simple freezing method (which involves sucrose preculture)
This method (Panis et al. 1996) is illustrated below.
|
The simple freezing method (figure: KULeuven/Bioversity) |
Cryopreservation
|
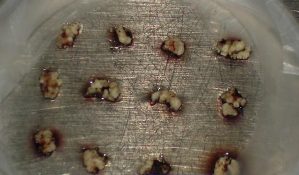
Precultured meristem clumps of "Musa schizocarpa"
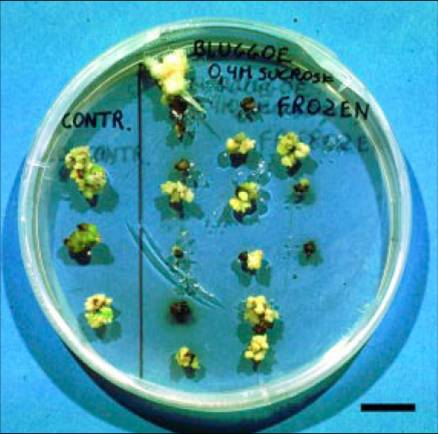
Petri dish containing control (left) and frozen (right) meristematic clumps of the cv. Bluggoe (ABB group), eight weeks after cryopreservation (bar = 1 cm) |
- Small white meristematic clumps weighing 5-15 mg (2-3 mm diameter), containing three to six meristematic domes, are excised from the precultured clumps. Brown tissues are removed and only the healthiest parts, as indicated by a white-yellowish colour, are retained.
- The clumps are transferred to sterile cryotubes (2 ml) without any liquid solution and plunged directly into a Dewar flask containing liquid nitrogen. Each cryotube contains 7-10 clumps. At this stage, samples can be stored for the long term by transferring the cryotubes to the liquid nitrogen tank, ensuring that their transfer from one container to the other takes place rapidly (within a few seconds), thereby preventing slow lethal re-warming of samples.
Re-warming and recovery
- After storage, rapid thawing takes place by stirring the frozen cryotube in a water bath at 40°C for 90 seconds.
-
Regeneration of the frozen meristems can be carried out in two different ways:
- Meristems are transferred to 9 cm Petri dishes containing semi-solid regeneration medium (P6) and sealed with parafilm.
- Alternatively, regeneration can be executed in liquid medium. Thawed meristems are transferred to 100 ml Erlenmeyer flasks containing 30 ml of liquid regeneration medium (P6 without solidifying agent) and placed on a rotary shaker at 70 rpm.
- After one week of culture in dark conditions, Petri dishes and flasks are transferred to continuous light at 50 µE m-2 s-1. Cultures are kept at all times at 25°C ± 2°C.
- Three weeks after transferring to the regeneration medium, frozen meristem regrowth is determined under a binocular microscope. Two types of surviving tissues are distinguished, i.e. shoots and non-regenerating callus. All calluses are systematically discarded and only recovered shoots (see photo on the right) are transferred to test tubes with regeneration medium to promote further development of whole plants. As soon as rooted plants are sufficiently developed, they are planted in soil.
Discussion and perspectives
The simple freezing protocol was applied to 36 banana cultivars belonging to 8 genomic groups (Panis et al. 2002). The results were extremely genotype dependent. Best results (up to 70% regrowth) have been obtained with the ABB cultivars like Bluggoe, Cachaco and Monthan. Intermediate results (around 25% regrowth) were reached with AAA dessert and AAB bananas. AAB plantains and diploids generally respond poorly. For all cultivars under investigation belonging to these genomic groups, plants were regenerated and grown in the greenhouse. However, most of the AAA Highland bananas were not able to withstand simple freezing.
With regard to this simple freezing method, blackening, due to the oxidation of polyphenols is often observed when re-warmed meristems are placed on semi-solid medium. This can cause cytotoxic effects and may also result in the recovering clumps being surrounded by an impermeable layer, thereby preventing nutrient uptake for further outgrowth. One method to overcome this problem is to use liquid regeneration media in order to dilute the released polyphenols. This resulted in regeneration percentages that were up to 20% higher
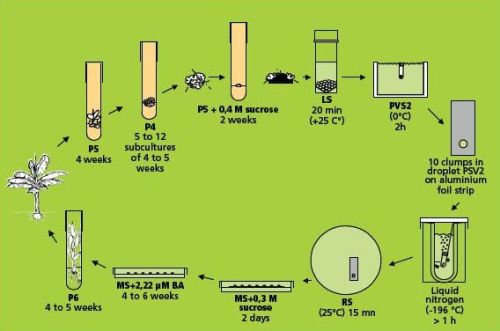
The droplet vitrification of ‘cauliflower-like’ meristem clusters method (combining simple freezing with droplet-vitrification)
This method (Panis et al. 2000, Agrawal et al. 2004) is illustrated in the figure below. Loading, dehydration, rapid freezing, storage, re-warming and unloading are almost identical to the droplet vitrification described in the page on Cryopreservation of banana apical meristems. Therefore, only the essential (and differential) steps are indicated in detail below.
|
Droplet vitrification protocol of meristem clumps (figure: KULeuven/Bioversity) |
Loading, dehydration and rapid freezing
|
Meristem clumps in a droplet of PVS2 solution (of about 15 μl) on a strip of aluminium foil (5x20 mm) |
- The excised sucrose precultured meristem clumps are left in the loading solution (see Appendix 1) in a 20 ml plastic vessel until all of them are dissected. The exposure time thus varies between 20 min. and 3 hrs.
- After loading, the solution is replaced by ice-cooled PVS2 solution (see Appendix 1). The meristem clumps are subjected to the PVS2 solution for 2 hrs. at 0°C. Five min before the end of the treatment, about ten meristem clumps are transferred to one droplet of PVS2 solution on a strip of aluminium foil (5x20 mm) with a forceps and a 2 ml plastic Pasteur pipette (see photo).
- To keep the temperature of the strip around 0°C during the manipulations, it is placed in a plastic Petri dish placed on top of a frozen cooling element. After the PVS2 treatment, the aluminium strip is plunged into liquid nitrogen with a fine forceps. For permanent cryostorage, the frozen foil is quickly transferred to a 2 ml cryotube filled with liquid nitrogen and closed.
Storage, re-warming and unloading
- The meristem clumps are kept in liquid nitrogen for at least 20 min. For re-warming, aluminium foil strips are plunged in 10 ml unloading solution (see Appendix 1) in a small Petri dish at room temperature. After a few seconds, meristem clumps are released from the aluminium foil and kept for another 15 min in the unloading solution. As such, the toxic PVS2 solution is removed whilst re-warming and replaced by a less-toxic unloading solution.
Recovery
- Frozen meristem clumps are taken from the unloading solution and placed in 9 cm plastic Petri dishes on two sterile filter papers on top of about 25 ml semi-solid hormone-free MS medium containing 0.3 M (= 102.6 g/L) sucrose.
- After two days, the filter paper is removed and the meristem clumps are transferred to Petri dishes with MS medium supplemented with 2.22 μM BA. The first week of culture always takes place in the dark.
- After a maximum of six weeks, meristem clumps are transferred to test tubes with P6 medium for further development of whole plants (see photos below).
 |
 |
|
Regenerating shoots from one control and three frozen proliferating meristems of the cultivars ‘Kisubi’ (AB group) ‘Dominico Harton’ (AAB plantain), ‘Bluggoe’ (AAB group) and ‘Williams’ (AAA group) three months after cryopreservation (photos: KULeuven/Bioversity) |
|
Discussion and perspectives
It has been found that post re-warming regrowth percentages of sugar pregrown meristems are higher compared to those grown on normal P4 medium. Sucrose preculture seems to increase tolerance of meristems not only towards the PVS2 solution but also towards the damaging events taking place during the cooling process. When comparing the results from the droplet vitrification of ‘cauliflower-like’ meristem clusters with those obtained using the simple freezing method for the same cultivar, an increase in viability percentages for almost all cultivars is observed. The increase in post-thaw regeneration for the ABB bananas is limited. Recovery remains between 50 and 70%. For AAA dessert and AAB bananas, the increase of regeneration percentages amount to 30-50%, while for plantains 20-30% is reached. AAA Highland bananas which proved to be recalcitrant towards cryopreservation using simple freezing give 0-20% survival using the droplet vitrification method.
For most plant species, optimal dehydration of meristematic tissues with PVS2 is obtained after 10-30 min. at room temperature (Takagi 2000). Among the exceptions are shoot apices of sweet potato and pineapple which need to be treated with PVS2 for 100 min. and 7 hrs., respectively (Plessis and Steponkus 1996, Gonzalez-Arnao et al. 1998). The duration of this treatment has to be optimized case by case since enough dehydration must take place to avoid the formation of lethal ice crystals during freezing. At the same time care has to be taken to prevent the treatment with the potentially toxic solution from irreversibly damaging the tissue. In the case of sucrose precultured proliferating banana cultivars, it has been observed that optimal post re-warming regeneration percentages are generally obtained after a 2 or 2.5 hr. PVS2 treatment. Survival after 3 hrs for most cultivars is considerably lower, probably due to the toxicity of this highly concentrated solution.
References and further reading
Agrawal A, Swennen R, Panis B. 2004. A comparison of four methods for cryopreservation of meristems in banana (Musa spp.). CryoLetters 25:101-110.
Dhed’a D, Dumortier F, Panis B, Vuylsteke D, De Langhe E. 1991. Plant regeneration in cell suspension cultures of the cooking banana cv. ‘Bluggoe’ (Musa spp., ABB group). Fruits 46 (2):125-135.
Gonzalez-Arnao MT, Ravelo MM, Villavicencio CU, Montero MM, Engelmann F. 1998. Cryopreservation of pineapple (Ananas comosus) apices, CryoLetters 19: 375-382.
Panis B. 2009. Cryopreservation of Musa germplasm: 2nd edition. Technical Guidelines No. 9 (F. Engelmann and E. Benson, eds). Bioversity International, Montpellier, France. Available here.
Panis B, Schoofs H, Thinh NT, Swennen R. 2000. Cryopreservation of proliferating meristem cultures of banana. In: Engelmann F, Takagi H, editors. Cryopreservation of tropical plant germplasm. Current research progress and application. Japan International Research Center for Agricultural Sciences, Tsukuba, Japan / International Plant Genetic Resources Institute, Rome, Italy. pp. 238-243.
Panis B, Strosse H, Van den Hende S, Swennen R. 2002. Sucrose preculture to simplify cryopreservation of banana meristem cultures. CryoLetters 23:375-384.
Panis B, Totté N, Van Nimmen K, Withers LA, Swennen R. 1996. Cryopreservation of banana (Musa spp.) meristem cultures after preculture on sucrose. Plant Science 121:95-106.
Plessis P, Steponkus PL. 1996. Effect of preculture in a sucrose-containing medium on the sugar content of potato shoot-tips, Cryobiology 33: 654-655.
Schoofs H. 1997. The origin of embryogenic cells in Musa. Dissertationes de Agricultura 330. Katholieke Universiteit Leuven, Belgium. 257 pp.
Strosse H, Schoofs H, Panis B, André E, Reyniers K, Swennen R. 2006. Development of embryogenic cell suspensions from shoot meristematic tissue in bananas and plantains (Musa spp.). Plant Science 170:104-112.
Strosse H, André E, Sági L, Swennen R, Panis B. 2008. Adventitious shoot formation is not inherent to micropropagation of banana as it is in maize. Plant Cell Tissue and Organ Culture 95:321-332.
Takagi H. 2000, Recent developments in cryopreservation of shoot apices of tropical species. In: Engelmann F, Takagi H, editors. Cryopreservation of tropical plant germplasm. Current research progress and application. IPGRI, Rome, Italy. pp. 178-193.
Comments
- No comments found







Leave your comments
Post comment as a guest