CGKB News and events Rice
Conservation of rice genetic resources
Contributors to this page: T.T. Chang Genetic Resources Centre-IRRI, Los Baños, Philippines (Ruaraidh Sackville Hamilton, Ken McNally, Flora de Guzman, Renato Reaño, Soccie Almazan, Adelaida Alcantara, Elizabeth Naredo); WARDA, Cotonou, Benin (Ines Sánchez); UPLB-University of the Philippines at Los Baños (Teresita Borromeo).
 Diversity of rice seeds (photo: IRRI) |
Importance of rice conservation
Ex situ conservation is a safe and efficient way to conserve rice genetic resources and to make the germplasm readily available to breeders and other researchers.
Traditional rice varieties and the wild rice species are being lost through genetic erosion. The farmers often adopt modern, new rice varieties (that produce more grain in less time) and no longer grow the traditional varieties they have been using for generations. When farmers switch from cultivating rice to growing other crops, the old rice seeds are also often forgotten. Eventually many of these traditional rice varieties are lost. Wild rice species are particularly threatened with extinction as their habitats are destroyed by human disturbance and by the rapidly changing and growing world. Future crop improvement needs the genetic variation of these traditional varieties and related wild genera to cope with the many biotic and abiotic stresses that are challenging rice production for both current and future generations.
Major rice collections
More than 219 000 accessions of cultivated and wild rice are stored in genebanks in more than 40 countries. Many of these accessions are duplicates, therefore this number does not necessary reflect the genetic diversity of this crop.
Currently the major rice collections in the CGIAR are maintained by the International Rice Research Institute (IRRI) and Africa Rice Center (AfricaRice). Centro Internacional de Agricultura Tropical (CIAT) maintains a working collection used for breeding rice for Latin American countries and the International Institute for Tropical Agriculture (IITA) provides the infrastructure in which the long-term collection of WARDA is housed.
IRRI has the global mandate to conserve and improve germplasm, while the other institutes have regional or continental mandates in Africa and Latin America.
 |
 |
|
Medium-term conservation of rice (photo: WARDA) |
Long-term conservation of rice (photo: WARDA) |
Safety duplication of cultivated rice, wild rice and related genera genetic resources
Contributors to this page: T.T. Chang Genetic Resources Centre-IRRI, Los Baños, Philippines (Ruaraidh Sackville Hamilton, Ken McNally, Flora de Guzman, Renato Reaño, Soccie Almazan, Adelaida Alcantara, Elizabeth Naredo); WARDA, Cotonou, Benin (Ines Sánchez); UPLB-University of the Philippines, Los Baños (Teresita Borromeo).
|
IRRI ships 35.5 million seeds to Svalbard |
|
 |
|
|
After months of preparation by staff of the Genetic Resources Center at IRRI, a replicate set of the Global Rice Collection from 123 countries held by IRRI left for the Svalbard Global Seed Vault or 'Doomsday Vault'. IRRI is contributing the largest number of accessions for a crop (70 180) and the largest estimated number of seeds (35 577 576). The video shows GRC staff members preparing and loading seed containers. Click to see the video on YouTube. |
When should it be used
- A safety back up should be kept of all unique accessions, under ideal conditions for secure long-term conservation, at least as good as the base collection.
- The safety back up should always be in a distant high-quality genebank, preferably in a different continent from the active and base collections (ensuring that the safety back up is subject to different risks minimizes the risk of simultaneous loss of safety back up, base and active collections).
Current best practices are for two levels of safety backup:
- A primary back up in a high-quality genebank chosen by the genebank holding the active collection (see general page on safety duplication for more details).
- A second, 'ultimate' back up in the Svalbard Global Seed Vault (click here and here for details).
- The ideal safety backup is a 'black box', remaining legally under the administration of the genebank providing the samples, not the genebank holding the safety back up.
- As black-box conservation in a remote genebank, seed packets should be packed into strong, secure, sealed boxes for shipment and should remain in the same boxes for storage in the remote store.
- Samples conserved in the safety back up collection should never be used, even for viability testing, except to restore samples in the event of the accidental loss of all samples in the active and base collections.
- Since the back up samples should not be used for testing viability, special consideration should be given to the need for monitoring viability in the safety back up collection.
- If the conditions of the backup storage are indubitably as good as or better than those of the base collection, there should be no separate viability monitoring schedule for the safety duplicate; monitoring viability in the base collection is used as a surrogate. However, if there is any doubt, additional samples should be deposited in the same location as the safety back up specifically to provide seed for viability testing.
Sample specifications
Minimum sample size for storage
- 15 g (enough for one planting) (see also general page on safety duplication).
Viability for storage
- 85% of initial viability.
Moisture content
- 6-7%.
Container specifications
Seed packaging method
Preparing seed packets
- Routine preparation of samples for safety back up should be undertaken at the same time and in the same place as for the base collection.
- Separate preparation of the safety back up is needed only in the case that a base collection has been established without arranging for safety duplication.
- Use a specially designated seed packing, labelling and weighing room, situated adjacent to the seed drying room and with humidity reduced using a dehumidifier.
- Prepare two small high-grade aluminium foil pouches per accession (one for the primary back up, one for Svalbard).
- Prepare barcoded labels for each packet, with the following printed information: accession number, name, crop year, Seed Lot ID, and barcode. The barcode itself should contain the Seed Lot ID.
- Prepare small paper labels with accession ID and crop year to be placed inside the packets.
- Label the pouches inside and outside, and arrange them in the packing area in the same order in which they will be withdrawn from the drying room.
- Take only a few samples at a time from the drying room.
- For each sample, find its corresponding labelled pouches and double check that all labels match.
- Pour at least 100 grains of seed into each pouch and seal using a high temperature constant heat sealer with 1 cm seal width.
- Place in temporary storage until sufficient samples have accumulated for shipping to the safety back up location.
Preparing containers for seed packets, to pack final container for shipping and storage
- Packing in parallel with the base collection improves efficiency and ensures that safety back up is a routine element of conservation.
- Label the container with a unique container ID.
- Sort foil pouches by expected date of replacement: divide them into groups by expected longevity and sort within groups by year of seed production.
- Divide the box into compartments with thin cardboard dividers, each compartment 10 cm wide x 12 cm tall x the length of the box (packing foil pouches into fixed rows 1 pouch wide and 1 pouch tall keeps them in place).
- Pack pouches tightly within each compartment, alternating pouches high and low (tight packing prevents dislodgment during transit; alternating high/low positions enables tighter packing given the central bulge of seed in each packet).
- Prepare box inventory with ID and layer of each accession in the box.
- Place printed copy of the inventory in the box.
- Seal the box for shipment.
Specifications of packaging material
Seed packets
- High grade laminated aluminium foil with wide seam on all sides, 12 cm x 9 cm.
- To ensure suitability for long-term conservation, use special-purpose pouches designed in consultation between the Global Crop Diversity Trust and the manufacturers – the specification is higher than for the pouches used for the active collection.
- Special-purpose high-quality labels created with material, adhesive and print designed to survive 100 years at 0-100% humidity and -20°C to +30°C, e.g. from CILS International.
Shipping/storage containers
- The containers must be strong enough to endure shipping, able to withstand extremes of temperature and moisture, of a size convenient for handling and storage.
- More detailed specifications are available from the Nordic Genetic Resource Centre for boxes destined for storage in the Arctic Seed Vault in Svalbard (click here).
Storage specifications
Assigning location codes in boxes
- Depending on the selected size, each box will have packets arranged probably in two layers of three long rows.
- Record the layer in which each accession is to be found (since packets are packed in order shown in the packed printer inventory, it is sufficient to record just the layer within the box occupied by each accession, without further information on where it is within the layer).
Storage conditions
- -18 to -20oC.
Shipping method
- Airfreight with temperature logger.
Legal arrangements
- Ensure there is a legal contract (typically a Letter of Agreement or similar) between the two parties concerned, detailing the rights and obligations of each party.
- Special agreement for Svalbard.
All shipments must be accompanied by
- Export permit (if required by the exporting country).
- Import permit (if required by the importing country).
- Customs declaration (if required by the importing country).
- GMO declaration (if required by the importing country).
- Phytosanitary certificate (if required by the importing country and no waiver is granted).
Recording information during safety duplication
The following information should be recorded for each step:
- Accession ID (ID of accession).
- Seed lot ID (ID of this sample of the accession).
- Crop season (year and season the seed was harvested).
- Storage type (primary safety backup, secondary safety backup, type of storage and packing for this sample).
- Location in storage (box and layer number).
- Date shipped (date when the sample was shipped to genebank holding safety backup collection).
- Species.
- Germination % (2 replications) (germination % - separate values for each replication; new record for each test).
- Germination test N (number of seed per replicate used for testing germination %).
- Germination date (date of germination test; new record for each test).
- Seed amount units – grams, number of packets (units used to record the amount of seed of this sample).
- Seed amount (amount of seed in store; new record for each update).
- Inventory date (date the amount was last updated; new record for each update).
References and further reading
Agreement between (Depositor) and the Royal Norwegian Ministry of Agriculture and Food Concerning the Deposit of Seeds in the Svalbard Global Seed Vault. Available [online] from: http://www.nordgen.org/sgsv/scope/sgsv/files/SGSV_Deposit_Agreement.pdf. Date accessed: 10 July 2013.
Characterization of cultivated rice, wild rice and related genera

Mature open panicle (photo:IRRI) |
Contributors to this page: T.T. Chang Genetic Resources Centre-IRRI, Los Baños, Philippines (Ruaraidh Sackville Hamilton, Ken McNally, Flora de Guzman, Renato Reaño, Soccie Almazan, Adelaida Alcantara, Elizabeth Naredo); WARDA, Cotonou, Benin (Ines Sánchez); UPLB-University of the Philippines at Los Baños (Teresita Borromeo).
|
Contents: |
Planting and cultural practices for characterization
For details on cultivated rice, click here.
For details on wild rice and related genera, click here.
Morphological descriptors for characterization
See list for rice descriptors developed by Bioversity International, IRRI and AfricaRice, (2007) and key access and utilization descriptors for rice genetic resources developed by Bioversity International and International Rice Research Institute (IRRI) (2009).
Pictures for characterization
Sufficient detail should be captured in images to taxonomically identify the plant and demonstrate the traits that show variation.
 From paddy (left) to brown rice (center) to polished rice (right) (photos: IRRI) |
- Take images for character(s) which may be difficult to describe verbally.
- Intact inflorescence.
- Whole grain as harvested (‘paddy’).
- De-hulled unpolished caryopsis (‘brown rice’).
- Polished rice.
- Store in a database file linked to other characterization data.
Herbarium samples for characterization
Use only for wild rice and related genera, where it is useful to have references.
- Sufficient detail should be captured to taxonomically identify the plant and demonstrate the traits that show variation.
- Take and store vouchers for future reference and taxonomic verification.
- Collect plants with leaves, flower spikes and seeds as well as roots.
- Spread plants open before pressing.
Molecular descriptors for characterization
Prior/current:
- Selected SSR markers with allelic controls (protocols for SSR marker technology are established; however, accurate scoring across diverse germplasm presents significant difficulties).
Current/near future:
- SNP genotyping platforms (SNPs arrays have been established, removing issues with scoring quality, and are replacing SSRs as the preferred technology).
Cytological characterization
- Not useful for cultivated rice, which are all diploid with AA genome (in contrast with wild rice species which have a range of genomes).
- Wild rice species are all either diploid (2n=24) or tetraploid (2n=48). Chromosome counts are a valuable aid to taxonomic identification.
- Wild rice species have chromosomes with recognizably different morphologies, classified as AA, BB, ..., KK.
Nutritional traits for characterization
- See list for rice descriptors developed by Bioversity International, IRRI and AfricaRice, 2007.
Recording information during characterization
- See list for rice descriptors developed by Bioversity International, IRRI and AfricaRice, 2007.
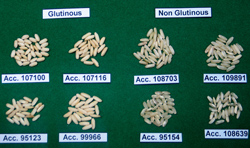 |
 |
|
Grain diversity: grains from Laos |
Showing the different stages of maturity of black caryopsis (photo: IRRI) |
References and further reading
Bioversity International, IRRI. 2009. Key access and utilization descriptors for rice genetic resources. Bioversity International, Rome, Italy; International Rice Research Institute, Philippines. Available here.
Bioversity International, IRRI and WARDA. 2007. Descriptors for wild and cultivated rice (Oryza spp.). Bioversity International, Rome, Italy; International Rice Research Institute, Los Banos, Philippines; WARDA, Africa Rice Center, Cotonou, Benin. Available here. (1.2 MB)
Borromeo TH, Sanchez PL, Vaughan DA. 1994. Wild rices of the
Chakravarthi BK, Naravaneni R, 2006. SSR marker based DNA fingerprinting and diversity study in rice (Oryza sativa L.) [online]. Available from: http://ajol.info/index.php/ajb/article/viewFile/42772/26341. Date accessed: 10 July 2013.
Chang TT, Vaughan DA.1989. Conservation and potentials of rice genetic resources. In: Bajaj YFS, editor. Biotechnology in agriculture and forestry.
Hanson J. 1985. Practical Manuals for Genebanks: Procedures for handling seeds in genebanks. IBPGR, Rome, Italy. HTML version available from: http://www2.bioversityinternational.org/publications/Web_version/188/. Date accessed: 10 July 2013.
IRRI. 2000. Manual of operations and procedures of the International Rice Genebank. Genetic
Korzun V. Molecular markers and their application in cereals breeding [online]. Available from: http://www.fao.org/biotech/docs/Korzun.pdf. Date accessed: 10 July 2013.
Lu BR. 1999. Taxonomy of the genus Oryza (Poaceae): Historical perspective and current status. IRRN 24.3. IRRI, Los Baños, Laguna.
Micro satellite markers [online]. Available from: http://www.gramene.org/markers/microsat/. Date accessed: 10 July 2013.
McNally KL, Bruskiewich R, Mackill D, Buell RC, Leach JE and Leung H. 2006. Sequencing multiple and diverse rice varieties. Connecting whole-genome variation with phenotypes. Plant Physiology 141, 26-31. Available from: http://www.plantphysiol.org/content/141/1/26.full. Date accessed: 15 July 2013.
Naredo MEB, Juliano AB, Lu BR, de Guzman FC, Jackson MT. 1998. Responses to seed dormancy breaking treatments in rice species (Oryza L). Seed Science and Technology 26:675-689.
Rao NK, Jackson MT. 1996a. Seed longevity of rice cultivars and strategies for their conservation in genebanks. Annals of Botany 77:251–260.
Rao NK, Jackson MT. 1996b. Seed production environment and storage longevity of japonica rices (Oryza sativa L.). Seed Science Research 6:17–21.
Rao NK, Jackson MT. 1996c. Effect of sowing date and harvest time on longevity of rice seeds. Seed Science Research 7:13–20.
Rao NK, Hanson J, Dulloo ME, Ghosh K, Nowel D, Larinde M. 2006. Manual of seed handling in genebanks. Handbooks for Genebanks No. 8. Bioversity International, Rome, Italy. Available in English (1.5 MB), Spanish (1.4 MB) and French (1.9 MB).
Reaño R, Pham JL. 1998. Does cross-pollination between accessions occur during seed regeneration at the International Rice Genebank. International Rice Research Notes 23(3):5-6.
Reed BM, Engelmann F, Dulloo ME, Engels JMM. 2004. Technical guidelines for the management of field and in vitro germplasm collections. Handbook for Genebanks No. 7. IPGRI, Rome, Italy. Available here.
Sackville-Hamilton NRS, Chorlton KH. 1997. Regeneration of accessions in seed collections: a decision guide. Handbook for Genebanks No. 5. IPGRI, Rome, Italy. Available here.
Sarao NK, Vikal Y, Singh K, Joshi MA, Sharma RC. 2009. SSR marker-based DNA fingerprinting and cultivar identification of rice (Oryza sativa L.) in Punjab state of India.
Tateoka T. 1962a. Taxonomic studies of Oryza I. O. latifolia complex. Bot. Mag. Tokyo 75:418-427.
Tateoka T. 1962b. Taxonomic studies of Oryza II. Several species complexes. Bot. Mag. Tokyo. 75:455-461.
Tateoka T. 1963. Taxonomic studies of Oryza III. Key to the species and their enumeration. Bot. Mag. Tokyo. 76:166-173.
van Soest LJM. 1990. Plant Genetic Resources: Safe for the future in genebanks. Impact of Science on Society 158:107-120.
Vaughan DA. 1989. The genus Oryza L. Current status of taxonomy. IRRI Research Paper Series 138,
Vaughan DA, Sitch LA. 1991. Gene flow from the jungle to farmers. Bioscience, Vol. 41(1):22-28.
Vaughan DA. 1992. The wild relatives of rice: A genetic resources handbook. IRRI,
Vaughan DA, Chang TT. 1992. In situ conservation of rice genetic resources. Economic Botany 46(4):368-383.
Vaughan DA, Morishima H, Kadowaki K. 2003. Diversity in the Oryza genus. Current Opinion 6:139-146.
Cultural practices for characterization of cultivated rice
Contributors to this page: T.T. Chang Genetic Resources Centre-IRRI, Los Baños, Philippines (Ruaraidh Sackville Hamilton, Ken McNally, Flora de Guzman, Renato Reaño, Soccie Almazan, Adelaida Alcantara, Elizabeth Naredo); WARDA, Cotonou, Benin (Ines Sánchez); UPLB-University of the Philippines at Los Baños (Teresita Borromeo).
Planting and cultural practices
Environment
 Characterization trial of rice (photo: IRRI) |
- For large diverse collections encompassing varieties from different ecosystems, use irrigated lowland with good drainage (this provides a single common environment suitable for growing and comparing almost all varieties, including upland, lowland and deep water varieties, regardless of their normal ecosystem and adaptation).
- For collections targeted to particular ecosystems, use the corresponding ecosystem (matching the ecosystem to the target provides more realistic characterization of varieties adapted to that ecosystem).
Note: In rice agriculture, the terms ‘upland’ and ‘lowland’ conventionally refer to the type of cultivation, not to the elevation of the land. Thus it is possible (and common) to have ‘upland’ rice in the lowlands and ‘lowland’ rice in the uplands.
‘Upland’ = on sloping land never inundated in water.
‘Lowland’ = on level ground periodically inundated, by rainfall or by irrigation water, to control weeds.
Soil type
- Silt clay to clay loam (best for lowland rice cultivation).
Rainfall
- Medium-high, or irrigate (rice does not grow well under drought; it needs either abundant rainfall or effective irrigation).
Season
- As appropriate to rice cultivation in the region.
- In tropical areas with two cropping seasons per year, select one season per year for characterization: usually the season that starts while days are growing longer.
- Long-duration varieties will extend into the second cropping season (using one fixed season each year improves consistency between trials.
- Some rice varieties are short-day photoperiod sensitive.
- In tropical latitudes higher than about 10°, the sensitivity to photoperiod (a key trait) can be tentatively characterized without multiple treatments by planting as the days are growing longer (e.g. in April-May in the northern hemisphere, October-November in the southern hemisphere); photoperiod sensitive accessions then remain vegetative for a prolonged period until the days shorten again. However, this does not distinguish between photoperiod-sensitive and long-duration photoperiod-insensitive varieties).
Plot size
- 5 m2 - 3 rows 5 m long (minimum plot size providing one row for sampling and one border row on each side).
Sampling area/border area
- Sampling area - one central row.
- Border area - one row on the edge of each plot, i.e. two border rows between successive sampled rows (minimum area good enough to record required traits at minimum maintenance cost).
Plant density
- Single seedling per hill, 30 x 30 cm between hills (use wider spacing than common agricultural practice for improved recording of tillering ability).
Replications
- Normally unreplicated entries, control varieties are replicated every 150 entries (replication of only control varieties is sufficient for normal assessment of highly heritable morpho-agronomic traits; more economical and thus allowing the screening of more accessions).
- For special purpose characterization for more rigorous statistical analysis, full replication is necessary.
Standard check cultivars
- For large diverse collections spanning all types, use up to six control varieties from the main taxonomic variety groups (glaberrima, indica, japonica, aus) and morpho-agronomic groups (glutinous, semi-dwarf).
- For smaller collections targeted at a particular ecosystem or environment, use a smaller number of check cultivars as appropriate for the target ecosystem and environment.
Frequency of standard checks
- Plant the full set of selected check varieties every 150 entries (this is the best practices considering the number of entries being handled and randomly scattered across the entire size of the field).
Time of day for data collection
- Field data are best collected in the morning (best time for consistent characterization of traits such as flowering that vary through and are most apparent in the morning. Best time also for the effectiveness of the observer).
- Post-harvest data can be taken any time, but for efficient use of time, good practice is to alternate field work in the morning with post-harvest characterization indoors in the afternoon.
Consult the general rice characterization page for information about descriptors, or recording of information during characterization.
References and further reading
Hanson J. 1985. Practical Manuals for Genebanks: Procedures for handling seeds in genebanks. IBPGR,
Rao NK, Jackson MT. 1996a. Seed longevity of rice cultivars and strategies for their conservation in genebanks. Annals of Botany 77:251–260.
Rao NK, Jackson MT. 1996b. Seed production environment and storage longevity of japonica rices (Oryza sativa L.). Seed Science Research 6:17–21.
Rao NK, Jackson MT. 1996c. Effect of sowing date and harvest time on longevity of rice seeds. Seed Science Research 7:13–20.
Rao NK, Hanson J, Dulloo ME, Ghosh K, Nowel D, Larinde M. 2006. Manual of seed handling in genebanks. Handbooks for Genebanks No. 8. Bioversity International, Rome, Italy. Available in English (1.5 MB), Spanish (1.4 MB) and French (1.9 MB).
Reaño R, Pham JL. 1998. Does cross-pollination between accessions occur during seed regeneration at the International Rice Genebank? International Rice Research Notes 23(3):5–6.
Reed BM, Engelmann F, Dulloo ME, Engels JMM. 2004. Technical guidelines for the management of field and in vitro germplasm collections. Handbook for Genebanks No. 7. IPGRI, Rome, Italy. Available here.
Sackville-Hamilton NRS, Chorlton KH. 1997. Regeneration of accessions in seed collections: a decision guide. Handbook for Genebanks No. 5. IPGRI, Rome, Italy. Available here.
Van Soest LJM. 1990. Plant Genetic Resources: Safe for the future in genebanks. Impact of Science on Society 158:107-120.
More Articles...
- Cultural practices for characterization of wild rice
- Seed bank for wild rice and related genera genetic resources
- Sample processing in seed banks of wild rice and related genera genetic resources
- Viability of wild rice and related genera genetic resources
- Storage of wild rice and related genera genetic resources
- Field bank for wild rice and related genera genetic resources
- Sample processing and viability in field banks (wild rice and related genera)
- Regeneration guidelines for wild rice and related genera
- Sample processing in seed banks of cultivated rice genetic resources
- In vitro bank (cultivated rice, wild rice and related genera)
Subcategories
-
main
- Article Count:
- 1
-
Regeneration
- Article Count:
- 3
-
Conservation
- Article Count:
- 1
-
Safety duplication
- Article Count:
- 1
-
Characterization
- Article Count:
- 3
-
Conservation - Wild Rice
- Article Count:
- 7
-
Conservation - Cultivated Rice
- Article Count:
- 13




