CGKB News and events Cassava
|
The importance and origin of Cassava |
|
|
How is it consumed?
How is it propagated? |
|
|
Further reading Carter, S.E., Fresco, L.O., Jones, P.G., Fairbairn, J.N.1997. Introduction and diffusion of cassava in Africa, IITA Research Guide 49. |
Cassava genetic resources
Contact persons for Cassava: Badara Gueye, IITA, Nigeria (in vitro), Soyode Folarin, IITA, Nigeria (field) or Daniel Debouck, CIAT, Colombia
Contributors to this page: CIAT, Colombia (Daniel Debouck); IITA, Nigeria (Dominique Dumet); Bioversity International/ILRI, Ethiopia (Alexandra Jorge); independent consultant (Clair Hershey); INIA, Peru (Llerme Rios).
Compilation of best practices
Information on best practices for genebank management of cassava genetic resources was gathered from current genebank practices and accumulated experience from CIAT and IITA, reviews from the literature and existing websites of major cassava genebanks (EMBRAPA, Brazil; INIA, Peru), as well as valuable information from independent cassava consultants. Crop experts revised and validated this information and these recommendations.
|
|
Cassava genetic resources are considered here to include all the species within the genus Manihot. This review focuses on cultivated cassava (Manihot esculenta) but also references the wild Manihot species in various sections. There are only a few genebanks world-wide that conserve wild Manihot species and the availability of information on best practices for genebank management of these species is still limited.
Importance and origin
Cassava (Manihot esculenta Crantz) is a perennial woody shrub with edible roots, which grows in tropical and subtropical areas of the world. It is also called yuca, manioc and mandioca, along with many other local names. Cassava has the ability to grow on marginal lands, especially in drought-prone conditions and in low fertility, acid soils, where cereals and other crops do not thrive. Because cassava roots can be stored in the ground (while still intact on the growing plant) for up to 24 months or more, harvest may be delayed until market, processing or other conditions are favourable.
The crop has been cultivated in tropical America for probably about 10 000 years. The origin of the species is unknown, but recent taxonomic and molecular evidence suggests a southern Amazon origin (Allem, 1987). It was introduced to Africa by Portuguese traders during the 16th century and later to Asia, both from Africa and from the Americas. It is now grown in over 90 countries and provides food and income for 500 million people in the developing world. It is estimated to be grown on 18.7 million hectares, yielding about 233 million tonnes of roots annually (FAOSTAT 2008).
Genetic resources
Genetic resources of cassava comprise local or introduced landraces, improved cultivars, genetic stocks and related wild species. All known cultivars of cassava belong to the species M. esculenta. According to the latest monograph, the genus Manihot contains 98 species (Rogers and Appan, 1973). The species are concentrated in Meso-America and Brazil, but are distributed widely in the Americas, from the US to Argentina. The genus is morphologically very diverse, containing herbs, shrubs and trees. Only cassava is cultivated. The wild species are mostly seed-propagated and many are very difficult to propagate vegetatively. Most of the species are shade-intolerant and tend to be invaders of forest borders or of open areas where they do not need to compete with other species for sunlight. Many produce storage roots, where starch reserves accumulate to help the plant endure difficult periods, especially drought.
|
|
Utilization
Cassava’s starchy roots are the basis of many food and industrial products. The roots can be eaten in various forms: raw, roasted, boiled, baked, fried, granules, pastes and flour. In many of the cassava-growing countries in Africa, as well as a few other countries of the world, the leaves are also consumed as a green vegetable, which provide protein and vitamins A and B.
All known cultivars produce cyanogenic glucosides in the roots. These glucosides combine with enzymes after cell disruption to release hydrogen cyanide. Only those cultivars with low cyanogenic potential are safe to eat after simple boiling. Many types of processing, such as fermentation, grinding, drying or roasting eliminate most of the cyanide. Through these various types of processing, cultivars of high cyanogenic potential can be rendered safe for human consumption.
The exact evolutionary benefits of high cyanogenic potential are unknown. There are few defined relationships between cyanogenic potential and pest resistance, for example. However, it is known that high potential can deter wild mammals from damaging the crop.
In large areas of subSaharan Africa, and in parts of Latin America and SE Asia, cassava provides a major basic daily source of dietary energy. Once harvested, the cassava roots spoil quickly and must be eaten or processed, typically within three to seven days, to preserve their food or nutritional value. Processing generally involves cooking, grinding, drying or fermenting, or some combination of these, which varies according to local customs. There are now promising results of good genetic variation for post-harvest deterioration and the possibility to improve this trait through breeding.
Cassava is used for human consumption, animal feed and a range of industrial products in Latin America. In Asia cassava has taken on primarily an industrial role. Nonetheless, it is still important as a human food in some Asian countries, especially in Indonesia. Cassava starch is used in a wide range of products: for example, in various food products, in pharmaceuticals, as a binding agent, in the production of paper and textiles and as monosodium glutamate, an important flavouring agent in Asian cooking. In Africa, cassava flour is beginning to be used in partial substitution for wheat flour.
Cassava has long been used as a component of balanced diets for animal feed and may substitute for maize, sorghum or barley depending on the commodity market. Normally a protein source such as soy needs to be added to cassava to obtain nutritional values similar in feed value to the cereal grains. The large cassava industry of Thailand developed out of a niche market for cassava chips and pellets in Europe's animal feed industry in the 1970s. Since the turn of the 21st century, use of cassava to produce ethanol has gained considerable popularity, especially in Asia.
Propagation
Cassava is mostly propagated vegetatively by stem cuttings. Its production is therefore greatly dependent on the supply and quality of these cuttings. The multiplication rate of this vegetative planting material is very low compared to grain crops, which are generally propagated by true seeds. Typically cassava has a multiplication rate on the order of 10:1, although this may vary widely depending on genotype and growing conditions. In addition to the constraint imposed by a low multiplication rate, cassava stem cuttings are bulky, difficult to transport and highly perishable: they may begin to dry out and lose viability within a few days after harvest. Moreover, phytosanitary regulations prohibit the movement of cassava stem cuttings across international borders (to prevent the spread of diseases and insects), so special arrangements have to be made for storage and transportation of germplasm.
Under natural conditions all the wild Manihot species are propagated from seed, although some can be propagated vegetatively under special care.
Networks and organizations
There are a number of networks and organizations that focus on cassava research and development, or include cassava among other commodities. During the 1970s and 1980s, several networks began and functioned for a period of time, but were not sustained mainly due to broad funding cuts for agriculture in the 1990s and 2000s. The remaining active organizations, among those listed below are: ISTRC, Africa Branch of the ISTRC, EARRNET and CLAYUCA.
African Branch of the ISTRC
African Francophone Cassava Network (CORAF)
Asian Cassava Research Network
Cassava R&D workers
Cassava Biotechnology Network
Cassava Genetic Resources Network
Collaborators in Root and Tuber Improvement and Systems (CORTIS)
Eastern and Southern African Root Crop Research Network (EARRNET) (http://www.asareca.org/earrnet/)
International Society for Tropical Root Crops (ISTRC) (http://www.istrc.org/)
Global Cassava Development Strategy (GCDS)
Global Cassava Partnership for Genetic Improvement (GCP-GI)
Latin American Consortium for Cassava (CLAYUCA) (http://www.clayuca.org/)
Latin American Integrated Projects Network
MOLCAS
Panamerican Cassava Breeders Network
Southern Cone Cassava Development Network
References and further reading
Allem AC. 1987. Manihot esculenta is a native of the neotropics. Plant Genetic Resources Newsletter 71:22-24. IBPGR/FAO.
Allem AC. 1994. The origin of Manihot esculenta Crantz (Euphorbiaceae). Genetic Resources and Crop Evolution 41:133-150. The Netherlands.
Allem AC. 2002. The origins and taxonomy of cassava. In: Hillocks RJ, Thresh JM, Bellotti AC, editors. Cassava: Biology, Production and Utilization. CABI, New York. pp. 1-16.
Best R, Henry G. 1994. Cassava: Towards the year 2000. In: International Network for Cassava Genetic Resources. Report of the First Meeting, CIAT, Cali, Colombia, 18-23 August 1992. International Crop Network Series No. 10, IPGRI, Rome, Italy. pp. 3-11.
Carter SE, Fresco LO, Jones PG, Fairbairn JN. 1997. Introduction and diffusion of cassava in Africa, IITA Research Guide 49.
Available from: http://old.iita.org/cms/details/trn_mat/irg49/irg49.html. Date accessed: 11 August 2010.
Ceballos H. 2006. Cassava research at CIAT [poster]. Centro Internacional de Agricultura Tropical (CIAT), Cali, Colombia. Available from: http://webapp.ciat.cgiar.org/news/pdf/poster02_scmeeting_06.pdf. Date accessed: 26 August 2010.
CIAT. 1984. Cassava Program Annual Report. Centro Internacional de Agricultura Tropical (CIAT), Cali, Colombia.
CIAT. 1993. Cassava Program Annual Report. Centro Internacional de Agricultura Tropical (CIAT), Cali, Colombia.
Devries J, Toenniessen G. 2001. Securing the harvest: biotechnology, breeding and seed systems for African crops. CABI Publishing, New York. 208 pp.
FAO/IFAD. 2000. The world cassava economy: facts, trends and outlook. FAO/IFAD. Rome. Available from www.fao.org/docrep/009/x4007e/x4007e00.htm. Date accessed: 25 March 2010.
FAOSTAT. 2008. Statistical database of the Food and Agriculture Organization of the United Nations FAOSTAT. Available from: http://faostat.fao.org. Date accessed: 10 August 2010.
Fresco L. 1986. Cassava in shifting cultivation: a systems approach to agricultural technology development in Africa. Royal Tropical Institute, Amsterdam. 290 pp.
Hahn SK, L Reynolds, GN Egbunike. 1992. Cassava as livestock feed in Africa: proceedings of the IITA/ILCA/University of IBADAN workshop on the potential utilization of cassava as livestock feed in Africa. 14-18 November, 1988. IITA. Ibadan, Nigeria. Available from: www.fao.org/Wairdocs/ILRI/x5458E/x5458E00.htm. Date accessed: 25 March 2010.
Hillocks RJ, Thresh JM, Bellott AC, editors. 2002. Cassava: biology, production and utilization. CABI International, NY. 332 pp.
Howeler RH, Oates CG, Allem AC. 2001. Strategic environmental assessment: an assessment of the impact of cassava production and processing on the environment and biodiversity. Proceedings of the Validation Forum on the Global Cassava Development Strategy, Rome, 26-28 April 2000. FAO and IFAD, Rome.
IITA. 1993. Annual Report. International Institute for Tropical Agriculture. Ibadan, Nigeria. 64 pp.
IPGRI. 1994. Report of the First meeting of the International Network for Cassava Genetic Resources, CIAT, Cali, Colombia, 18-23 August 1992. International Crop Network Series No. 10. International Plant Genetic Resources Institute, Rome, Italy. 179 pp.
Lozano JC, Byrne D, Bellotti A. 1980. Cassava/ecosystem relationships and their influence on breeding strategy. Tropical Pest Management. 26:180-187.
Machin D, Nyvold S, editors. 1992. Roots, tubers, plantains and bananas in animal feeding. In: Proceedings of the FAO expert consultation held in CIAT, Cali, Colombia. 21-25 January 1991. FAO. Rome. Available from: www.fao.org/DOCREP/003/T0554E/T0554E00.HTM. Date accessed: 25 March 2010.
Nweke FI, Spencer DSC, Lynam JK. 2002. The cassava transformation: Africa’s best-kept secret. Michigan State University Press, East Lansing, MI.
Olsen KM. 2004. SNPs, SSRs and inferences on cassava’s origin. Plant Molelucar Biology 56:517-526.
Ospina B, Ceballos H, editors. 2002. La yuca en el tercer milenio: sistemas modernos de producción, procesamiento, utilización y comercialización [Cassava in the third milenium: modern systems of production, processing, utilization and commercialization]. CIAT Publication No. 327, CIAT, Cali, Colombia.
Phillips T, Taylor D, Sanni L, Akoroda M. 2004. A cassava industrial revolution in Nigeria: the potential for a new industrial crop. IFAD, FAO, Rome. Available from: www.fao.org/docrep/007/y5548e/y5548e00.htm. Date accessed: 25 March 2010.
Purseglove JW. 1968. Tropical crops: dicotyledons. Longman, London.
Roa AC, Maya MM, Duque MC, Tohme J, Allem AC, Bonierbale MW. 1997. AFLP analysis of relationships among cassava and other Manihot species. Theoretical and Applied Genetics 95:741-750.
Rogers DJ, Appan SG. 1973. Manihot, Manihotoides (Euphorbiaceae), Flora Neotropica, Monograph no. 13. Hafner Press, New York. 272 pp.
Tewe OO. 2004. Cassava for livestock feed in sub-Saharan Africa. FAO/IFAD, Rome. Available from: www.fao.org/docrep/007/j1255e/j1255e00.htm. Date accessed: 25 March 2010.
Safety duplication of cassava genetic resources
Contributors to this page: Contributors to this page: CIAT, Colombia (Daniel Debouck); IITA, Nigeria (Dominique Dumet); Bioversity International/ILRI, Ethiopia (Alexandra Jorge); independent consultant (Clair Hershey); INIA, Peru (Llerme Rios).
When should it be used
- For all original germplasm collected by the genebank.
- For germplasm held only by the genebank.
- Safety duplicated material should preferably be located in a different country.
See also the general page on safety duplication procedures, as well the procedures on the safe transfer of clonal germplasm that should be carefully followed.
Cassava germplasm should be duplicated in a systematic way, consistently sharing information and germplasm to optimize the conservation system.
Material should be duplicated in more than one location and/or using more than one method (i.e. in the field and in vitro or tissue culture and cryo).
Sample specifications
- Minimum of 1-3 explants per accession.
Container specifications
To ensure explant viability in the genebank and en route to the users.
- Glass test tubes covered and sealed.
Storage specifications
Storage conditions
- As specified for slow growth storage.
Shipping method
- Prepare the documentation of outgoing plant material shipments with Phytosanitary Certificate, the Import Permit and the Standard Material Transfer Agreement (SMTA).
- Follow the same procedures for the plant material shipments from the genebank.
- Packages should be shipped using the most reliable, quickest and most cost effective route and carrier possible, in order to prevent deterioration of plant quality during transfer.
- Generally avoid shipments during the hottest times of the year.
Legal arrangements
The source centre, host country and importing centre and country policies and practices for germplasm movement for black-box storage must be fulfilled. The documentation listed below should be included to facilitate customs clearance:
- MOU for safety duplication.
- Phytosanitary Certificate.
- Certificate of origin.
- Certificate of no-commercial value.
- Electronic and hard copy of associated passport information.
- GMO-free certificate (if required).
IITA, Nigeria has the following procedure:
- An agreement with IITA-Cotonou to keep a black-box, including:
- Special agreement with the Plant Quarantine Services of Benin to use only an import permit and no need for Phytosanitary Certificates.
- Germplasm cannot be used in Benin or distributed elsewere.
- If material needs to be transferred to the field, it should first be repatriated to Nigeria.
CIAT, Colombia follows these procedures:
- An agreement with CIP, Peru to keep a black-box (non-active duplicate) of cassava germplasm that is only growing in vitro.
- Cassava core collections are also maintained as safety duplicates in liquid nitrogen (cryopreservation).
Recording information during safety duplication
The following steps should be taken regularly, due to the short-term duration of conserving clonal crops:
- Updated lists of existing germplasm should be sent to the owner of germplasm regularly (every 3-4 months in the case of IITA).
- Lists should be checked and any new material (not duplicated in the recipient genebank) should be identified.
- Depending on seedling availability at the owner’s genebank, seedlings should start to be prepared and the recipient genebank informed to start preparing the relevant documentation.
The following information should be kept updated:
- Number of seedlings per accession.
- Date of production of seedling.
- Date of safety duplication.
- Name of institute holding the safety duplicate.
- Box label where the sample is placed.
References and further reading
Frison EA, Feliu E, editors. 1991. FAO/IBPGR Technical Guidelines for the Safe Movement of Cassava Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Characterization of cassava genetic resources
Contributors to this page: CIAT, Colombia (Daniel Debouck); IITA, Nigeria (Dominique Dumet); Bioversity International/ILRI, Ethiopia (Alexandra Jorge); independent consultant (Clair Hershey); INIA, Peru (Llerme Rios); Bioversity International, Rome (Adriana Alercia).
Characterization of cassava germplasm (and many clonal crops) is particularly important for the following purposes:
- To verify the genetic integrity of material kept under the in vitro conservation technique (especially after several years of conservation).
- To detect mixtures of materials.
- To identify genetic duplication (redundancy of material).
|
Contents: |
Planting and cultural practices for characterization
Clonal crops are usually characterized during field regeneration. See also the regeneration page for the complete details of cultural practices.
|
|
Environment
- Material should be characterized in an environment that is highly suited to cassava growth and development, and where biological and environmental problems are minimized, so that there are no confounding effects on the expression of traits used for characterization.
Plot size
- Spacing between plants 1-1.5 m.
Sampling area/border area
- 2.2 m, 1.5 m and 1.0 m between adjacent plots for high, intermediate and low vigour groups (to allow the scoring of individual plots and avoid mixing by lodging).
Plant density
- Maximum 1 plant per m2.
Replications
- Normally unreplicated entries with control varieties regularly replicated (replication of only control varieties is sufficient for normal assessment of highly heritable morpho-agronomic traits; this practice is more economical and thus allows the screening of more accessions).
- For special-purpose characterization for more rigorous statistical analysis, full replication is necessary.
Standard check cultivars
- Local standard cultivars should be used as a reference, especially for pest and disease screening.
Frequency of standard checks
- Random or after every 20 accessions.
Morphological descriptors for characterization
The list of suggested descriptors below is from Fukuda et al. (2010), who developed a descriptor list for IITA. This is based on descriptors used by both CIAT (Colombia) and EMBRAPA (Brazil), and thus encompasses the world’s major cassava collections. The publication includes a full complement of colour photos to facilitate understanding of the states of each descriptor.
Descriptors to be scored at three months after planting
- *1. Colour of apical leaves.
- *2. Pubescence on apical leaves.
Descriptors to be scored at six months after planting
- 3. Leaf retention.
- *4. Shape of central leaflet.
- *5. Petiole colour.
- *6. Leaf colour.
- 7. Number of leaf lobes.
- 8. Length of leaf lobe.
- 9. Width of leaf lobe.
- 10. Ratio of lobe length to lobe width of central leaf lobe.
- 11. Lobe margins.
- 12. Petiole length.
- 13. Colour of leaf vein.
- 14. Orientation of the petiole.
- 15. Flowering.
- 16. Pollen.
Descriptors to be scored at nine months after planting
- 17. Prominence of foliar scars.
- 18. Colour of stem cortex.
- 19. Colour of stem epidermis.
- *20. Colour of stem exterior.
- 21. Distance between leaf scars.
- 22. Growth habit of stem.
- 23. Colour of end branches of adult plant.
- 24. Length of stipules.
- 25. Stipule margin.
|
|
Descriptors to be scored at harvest
- 26. Fruit.
- 27. Seed.
- 28. Plant height.
- 29. Height to first branching.
- 30. Levels of branching.
- 31. Branching habit.
- 32. Angle of branching.
- 33. Shape of plant.
- 34. Number of storage roots/plant.
- 35. Number of commercial roots/plant.
- 36. Extent of root peduncle.
- 37. Root constrictions.
- 38. Root shape.
- *39. External colour of storage root.
- *40. Colour of root pulp (parenchyma).
- *41. Colour of root cortex.
- 42. Cortex: ease of peeling.
- 43. Texture of root epidermis.
- 44. Root taste.
- 45. Cortex thickness.
- *46. Dry matter content.
- 47. Starch content
- 48. Harvest index.
- *49. Cyanogenic potential.
- *50. Postharvest deterioration.
- *Total fresh weight of storage roots per plant (not included in IITA descriptors).
*Descriptors in the list of key access and utilization descriptors developed by Bioversity International and CIAT (2009).
In addition to the above, any other local quality traits of importance could be included. New traits identified by industry should be considered for inclusion, such as the ratio of amylose to amylopectin.
Pictures for characterization
Sufficient detail should be captured in images to taxonomically identify the plant and demonstrate the traits that show variation.
- Take images for character(s) which may be difficult to describe verbally.
- Store in a database file linked to other characterization data.
Herbarium samples for characterization
- It is suggested they should be taken and stored for future reference during multiplication/regeneration, particularly for primitive and wild relatives (to verify the accession identity and to be used as reference material).
Molecular descriptors for characterization
- SSR, EST-SSR, AFLP, RAPD (these are the current more efficient techniques for genetic diversity analysis and to identify duplicates).
Cytological characterization
This type of characterization should be done in specialized laboratories. Cytological characterization, with current available techniques, is not likely to be broadly applied across all accessions of a collection, but might be targeted to sub-samples such as the core collection.
Recording information during characterization
- Reference to the trials (details of which will be stored in separate database with information on dates, place, trial layout, people in charge of trial, etc.).
- Fields selected from the cassava descriptor list.
References and further reading
Bertram RB. 1993. Application of molecular techniques to genetic resources of cassava (Manihot esculenta Crantz, Euphorbiaceae): interspecific evolutionary relationships and intraspecific characterization. University of Maryland. (PhD thesis).
Bioversity International, CIAT. 2009. Key access and utilization descriptors for cassava genetic resources. Bioversity International, Rome, Italy, International Center for Tropical Agriculture (CIAT), Cali, Colombia. Available here.
Fregene M, Vargas I, Ikea J, Angel F, Thome J, Asiedu RA, Akoroda MO, Roca WM. 1994. Variability of chloroplast DNA and nuclear ribosomal DNA in cassava (Manihot esculenta Crantz) and its wild relatives. Theor. Appl. Genet. 89:719-727.
Fukuda WMG, Guevara CL.1998. Descritores morfológicos e agronómicos para a caracterização de madioca (Manihot esculenta Crantz). [Morphological and agronomic descriptors for characterizing cassava (Manihot esculenta Crantz] Documentos CNPMF no. 78. EMBRAPA/CNPMF. Cruz das Almas BA, Brazil.
Fukuda WMG, Guevara CL, Kawuki R, Ferguson ME. 2010. Selected morphological and agronomic descriptors for the characterization of cassava. International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria. 19 pp. Available from: http://www.iita.org/c/document_library/get_file?uuid=4530a72e-917d-4801-9239-cb0ee3a4dd4e&groupId=25357. Date accessed: 30 September 2010.
Gulick P, Hershey C, Esquinas Alcazar J. 1983. Genetic Resources for cassava and wild relatives. Series AGPG:IBPGR/82/111. IBPGR Secretariat, Rome. 56 pp.
Iwanaga M, Ayala ME, Ocampo CH, Hershey C. 1993. Caracterización de la colección de Colombia del germoplasma de yuca (Manihot esculenta Crantz) por electroforesis PAGE utilizando la isoenzima aß-esterasa. In: Clausen AM, editor. Recursos genéticos hortícolas, Instituto Nacional de Tecnología Agropecuaria, Balcarce, Argentina. pp. 244-258.
Ocampo CH, Hershey C, Iglesias C, Iwanaga M. 1993. Esterase isozyme fingerprinting of the cassava germplasm collection held at CIAT. In: Roca WM, Thro AM, editors. Proceedings of the First International Scientific meeting of the Cassava Biotechnology Network (CBN), Cartagena, Colombia, 25-28 August 1992, Cali, Colombia: CIAT (Working Document No. 123). pp. 81-89.
Ocampo CH, Angel F, Jímenez A, Jaramillo G, Hershey C, Granados E, Iglesias C. 1995. DNA fingerprinting to confirm possible genetic duplicates in cassava germplasm. In: Roca WM, Thro AM, editors. Proceedings of the Second International Scientific meeting of the Cassava Biotechnology network. Bogor, Indonesia, 22-26 August 1994. Working Document No. 150. Centro Internacional de Agricultura Tropical (CIAT). Cali, Colombia. pp. 145-151.
Roca, WM, Rodriguez JA, Roa J. 1983. Procedures for Recovering Cassava and Sweet potato Germplasm Distributed In vitro. Genetic Resource Unit CIAT Apartado Aéreo 6713 Call, Colombia S.A . Available from: http://www.scribd.com/doc/35771901/Manual-for-TC-Cassava-Sweet-Potato. Date accessed: 27 August 2010.
Conservation of cassava genetic resources
Contributors to this page: CIAT, Colombia (Daniel Debouck); IITA, Nigeria (Dominique Dumet); Bioversity International/ILRI, Ethiopia (Alexandra Jorge); independent consultant (Clair Hershey); INIA, Peru (Llerme Rios).
Importance of cassava conservation
The potential for genetic improvement of any crop relies on the ability to successfully use the existing genetic resources, including the related wild species. Cassava is the fourth most important food calorie crop in the tropics, and is growing in importance both for food security (especially Africa) and in multiple commercial and industrial uses (mainly Latin America and Asia). The current cassava genetic resources are primarily landrace varieties of Manihot esculenta and wild Manihot species that evolved under natural selection, as well as farmer conditions (cultivated cassava) for centuries or even millennia. In the only comprehensive monograph on the genus, Rogers and Appan (1973) identified 98 species in the genus. Most of the genetic diversification occurred in the Americas where the genus originated, but secondary centers of diversity also exist in Asia and Africa.
It is estimated that nearly 2/3 of the genetic diversity exists only in situ, with only 1/3 being securely conserved ex situ. However, these estimates are based on expert opinion rather than on solid survey evidence and genetic analyses (Hershey, 2008).
- There are still many unique native landrace varieties to be collected and safely conserved.
On a global basis, cassava has relatively few improved varieties relative to many other important crops, since there are very few fully-fledged breeding programmes in the world, and most of these operate with very constrained resources. Most cassava germplasm in use is still based on landrace varieties. However, the adoption of new improved varieties has increased rapidly since the turn of the 21st century. In some cases, this adoption of new varieties is creating a risk for loss of native landraces.
|
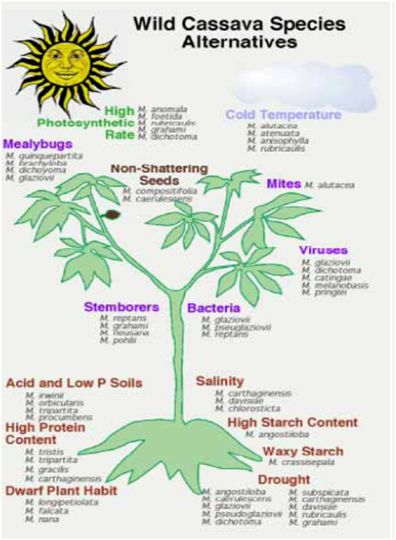
Figure 1 (Source: CIAT cassava programme) |
- Overall, farmers are adopting new varieties faster in Asia than elsewhere, especially in areas with high industrial demand. Landraces may be neglected and lost when adoption rates are high. Nonetheless, the areas where adoption rates are highest – China, Thailand and Vietnam – have very few landrace varieties and the development of new hybrids based on germplasm from the Americas is in fact introducing greater genetic diversity compared to what existed previously.
- In Africa, new varieties have been adopted due to increasing vulnerability of landraces to pests and diseases and higher productivity of new varieties. Landraces tend to disappear and many countries do not have adequate genebank conservation facilities.
- Expansion of agriculture and urbanization in South and Meso-America are shrinking the habitats of wild Manihot species.
- Wild species have played a limited but important role to date in cassava breeding programmes. However, the new challenges of cassava breeding, especially considering the new traits that will be needed for adaptation to climate change, and for new markets, are creating demand for new gene sources. This emphasizes and supports the need to collect and conserve wild types. To date there has been limited evaluation of the wild species for traits that may be relevant to the future of cassava breeding. Major efforts are needed to collect and conserve the Manihot species, especially those in areas where urbanization and agricultural expansion are threatening native habitats, such as in central Brazil.
- Figure 1 illustrates examples of the many possible traits that might be derived from wild Manihot species. However, it should be noted that most of these traits are hypothetical, as proper evaluations have not yet been done for the most part.
Conservation methods
Cassava
The combinations of genes currently found in the cassava landraces are a result of many centuries of farmers’ selection. Since cassava is a highly heterozygous crop, the only way to preserve these specific gene combinations is through vegetative propagation. In all cases, when seeds are produced from a particular landrace accession, there is broad segregation of the progeny due to the heterozygous nature of the parents. If accessions are to be recommended and used directly as new varieties by farmers, then it is critical that these specific gene combinations are preserved, for the desired traits to be expressed. However, if accessions are to be used by breeders as sources of specific genes, then the specific gene combinations are less important. In this case, seed conservation may be as good as, or better than, clonal conservation.
- The most widely used method of cassava conservation is through vegetative methods.
- Germplasm can be maintained vegetatively in fields or screenhouses (field banks) or in vitro (slow growth tissue culture or cryopreservation).
- When the objective is solely to conserve the genes (but not specific combinations of genes), true seed can be used instead, as long as flowering occurs. Seeds can also be conserved at low temperature and humidity or with cryo techniques.
- Genes can also be maintained for further use in the form of DNA (DNA banks) or cryopreserved pollen.
Genebanks need to have clear priorities as to which type of germplasm should be collected, efficiently conserved and securely duplicated.
- Small genebanks may be able to effectively and economically conserve all material, including accessions introduced from other countries and bred material.
- Larger genebanks should prioritize the conservation of local landraces, especially if resources do not allow adequate conservation of the entire collection that includes introduced material that is simultaneously conserved in other genebanks.
- If core collections have been defined, these may be given the highest priority, especially in terms of making material available for evaluation and breeding. Core collections are typically relevant only for large collections and there are only a few of these worldwide.
- Conservation of breeding lines and introductions should typically have lower priority than local material.
The conservation of local landraces in particular must be secured. Additional security can be provided by the following procedures:
- Duplicate in vitro collections in separate locations (preferred).
- Maintain a field bank and an in vitro bank.
- Use two separate field locations (least preferred).
Wild species
The wild Manihot species are conserved mainly in seed genebanks or as plants in the field. Since they are perennials, field genebanks may be maintained for long periods without regeneration if conditions are appropriate. Generally, the wild Manihot genebanks are planted or regenerated using seed. Some of the species have been successfully conserved and propagated in vitro; however each species appears to have unique optimum media and environmental conditions, and the research to define the suitable conditions has been done for only a relatively few species.
Major cassava collections
The largest ex situ cassava collections are held by CIAT-Colombia with about 6500 accessions, followed by EMBRAPA-Brazil (about 4000 accessions). Other important genebanks are those in CTCRI-India , INIA-Peru, NRCRI-Nigeria, IITA-Nigeria, IAN-Paraguay, SRCV-Benin, D.R. Congo and PGRC/CRI-Ghana.
The exact number of unique accessions can only be estimated, since there has not been an attempt to identify duplicate accessions across genebanks. There are probably more than 10 000 unique accessions conserved ex situ, in the more than 70 cassava genebanks worldwide (Hershey, 2008). Although there are no scientifically- or survey-based estimates, Hershey (2008) estimated more than 26 000 unique cassava landrace varieties, meaning that there are more uncollected landrace varieties than there are accessions already conserved ex situ. Clearly not every distinct genotype needs to be collected in order to capture the range of genetic diversity of the species. Hershey (2008) estimated that about 14 000 accessions, appropriately selected for genetic diversity, need to be collected and conserved to assure the long-term accessibility to the full range of genetic diversity of Manihot esculenta, and avoid genetic erosion of the species.
Many genebanks have part of their collection in tissue culture (in vitro). Among the major collections, only the CIAT genebank conserves the the collection exclusively in tissue culture or in cryo conditions. A few genebanks (mainly EMBRAPA, Brazil and CIAT, Colombia) have seed banks to conserve seeds of wild species or breeding material. A few are also initiating DNA banks.
CIAT and IITA are the two CGIAR centres sharing the international mandate for cassava research, breeding and improvement. They have been disseminating thousands of in vitro plantlets of improved varieties to dozens of countries through collaborating NARs or the private sector and development agencies. In addition, a very wide range of genetic diversity has been introduced from the CGIAR genebanks into national programmes through seed introductions. However, this is typically done by breeding programmes and does not necessarily become part of the cassava genebank.
The wild Manihot species have been given relatively little attention in terms of systematic collection and ex situ conservation. Firstly, many populations of these species have not been sampled and, secondly, many collected accessions have been lost due to difficulty in establishing and maintaining them.
References and further reading
Allem AC. 1992. Manihot germplasm collecting priorities. In: Roca WM, Thro AM, editors. Report of the First meeting of the International Network for Cassava Genetic Resources, 18-23 August 1992. Centro Internacional de Agricultura Tropical, Cali, Colombia. pp. 87-110.
Bonierbale M, Guevara C, Dixon AGO, Ng NQ, Asiedu R, Ng SYC. 1997. Cassava. In: Fuccillo D, Sears L, Stapleton P, editors. Biodiversity in Trust. Cambridge University Press. pp. 1-21.
Debouck DG. 1991. Genetic variation in crop species and their wild relatives: a viewpoint for their conservation. In: Becker B, editor. Genetic diversity and crop strategies for roots and tubers. Arbeitsgemeinschaft Tropische und Subtropische Agrarforschung e.V. and International Board for Plant Genetic Resources, Bonn, Germany, pp. 41-51.
Epperson JE, Pachico DH, Guevara CL. 1996. The cost of maintaining genetic resources of cassava, Manihot esculenta Crantz. Acta Horticulturae 429:409-413.
Epperson JE, Pachico DH, Guevara CL. 1997. A cost analysis of maintaining cassava plant genetic resources. Crop Science 37(5): 1641-1649.
Guevara C, Ospina JA, Mafla G, Verdier V. 1998. Zygotic embryo culture of Manihot esculenta Crantz: a practical approach for the safe international movement of cassava seed stocks. FAO/Bioversity Plant Genetic Resources Newsletter 115:33-38.
Gulick P, Hershey C, Esquinas AJ. 1983. Genetic resources of cassava and wild relatives. Series AGPG:IBPGR/82/111. IBPGR Secretariat, Rome. 60 pp.
Hershey C. 2008. A global conservation strategy for cassava (Manihot esculenta) and wild Manihot species. Summary of stakeholder deliberations and recommendations prepared for the Global Crop Diversity Trust. Available from: http://isa.ciat.cgiar.org/urg/urgweb_folder/files/unitfiles/A%20Global%20Conservation%20Strategy%20for%20Manihot%20%20August%202008-2.pdf. Date accessed: 7 Oct. 2010.
Hershey C, Iglesias C, Iwanaga M, Tohme J. 1994. Definition of a core collection for cassava. In International Network for Cassava Genetic Resources. Report of the First Meeting, CIAT, Cali, Colombia, 18-23 August 1992. International Crop Network Series No. 10, IPGRI, Rome, Italy. pp. 145-156.
Hidalgo R. 1995. Conservación Ex-Situ. In: "Memorias. Curso de Documentación de Recursos Fitogenéticos". Auspiciado por Universidad Nacional de Colombia, Bioversity y CIAT. Palmira. pp. 33-41.
Koo B, Pardey PG, Debouck D. 2004. CIAT genebank. In: Koo B, Pardey PG, Wright BD. Saving seeds: the economics of conserving crop genetic resources ex situ in the future harvest centres of the CGIAR. CABI Publishing, Wallingford, UK.
Leyton M. 1993. Crioconservación de pollen de yucca. Bachelor’s thesis. Univ. del Valle, Facultad de Ciencias, Dept. de Biología, Cali, Colombia.
Nassar NMA. 2006. Cassava genetic resources: extinct everywhere in Brazil. Genetic Resources and Crop Evolution 53:975-983.
Orrego JI, Hershey C. 1984. Almacenamieno del polen de yuca (Manihot esculenta Crantz) por medio de liofilizacion y varios regimenes de humedad y temperatura. [Storage of cassava (Manihot esculenta Crantz) pollen by means of lyophilization and various temperature and humidity regimes]. Acta Agronomica (Colombia) 34:21-25.
Patiño VM, Hershey CH. 1981. Collection priorities for cassava (Manihot esculenta) and wild Manihot species in Latin America. Series AGPG:IBPGR/81/95. IBPGR, Rome, Italy.
Perry M. 1994. Documentation and databases for germplasm collections. In International Network for Cassava Genetic Resources. Report of the First meeting, CIAT, Cali, Colombia, 18-23 August 1992. International Crop Network Series No. 10, IPGRI, Rome, Italy. pp. 157-162.
Rogers DJ, Appan SG. 1973. Manihot, Manihotoides (Euphorbiaceae), Flora Neotropica, Monograph no. 13. Hafner Press, New York. 272 pp.
Unnikrishnan M, Easwari Amma CS, Sreekumari MT, Sheela MN, Mohan C. 1987. Cassava germplasm conservation and improvement in India. In Proceedings of a cassava workshop in Asia. February 1987. Available from: http://webapp.ciat.cgiar.org/asia_cassava/pdf/proceedings_workshop_02/87.pdf. Date accessed: 26 August 2010.
More Articles...
- In vitro conservation of cassava genetic resources
- Cryo bank for cassava genetic resources
- Seed banks for cassava genetic resources
- Field banks for cassava and wild Manihot species
- Distribution of cassava genetic resources
- Viability and monitoring in cassava field banks
- Field management in cassava field banks
- Sample preparation in cassava field banks
- Slow growth storage (SGS) of cassava genetic resources
- Health diagnosis of cassava genetic resources
Subcategories
-
main
- Article Count:
- 1
-
Safety duplication
- Article Count:
- 1
-
Characterization
- Article Count:
- 1
-
Conservation
- Article Count:
- 11
-
Regeneration
- Article Count:
- 2