CGKB News and events Cassava
|
The importance and origin of Cassava |
|
|
How is it consumed?
How is it propagated? |
|
|
Further reading Carter, S.E., Fresco, L.O., Jones, P.G., Fairbairn, J.N.1997. Introduction and diffusion of cassava in Africa, IITA Research Guide 49. |
Distribution of cassava genetic resources
Contributors to this page: CIAT, Colombia (Daniel Debouck); IITA, Nigeria (Dominique Dumet); Bioversity International/ILRI, Ethiopia (Alexandra Jorge); INIA, Peru (Llerme Rios); independent consultant (Clair Hershey).
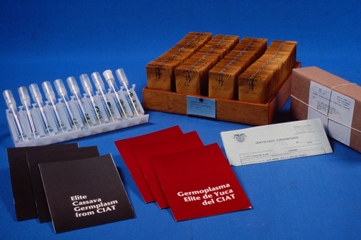
An important role of in vitro genebanks is the distribution of germplasm to different users.
Click here to open a PDF document that shows how to disseminate germplasm in detailed steps.
|
Contents: |
 Cassava dissemination (photo:IITA) |
Common policies on distribution and access to plant material
- Follow the International Treaty on Plant Genetic Resources for Food and Agriculture (ITPGRFA) (www.planttreaty.org) for in-trust germplasm using the Standard Material Transfer Agreement (SMTA).
Policy exceptions
- None.
National laws and regulations
It is essential to follow the terms and conditions in the host country agreements:
- Export permits.
- Phytosanitary certificate.
- Certificate of origin.
International laws and regulations
The shipment of plant material should be sent with the SMTA under the International Treaty for Plant Genetic Resources for Food and Agriculture.
Phytosanitary regulations
- Phytosanitary certificates are needed for most countries.
- See the safe transfer of germplasm (STOG) section in this website (this is essential to avoid the spread of pests and diseases).
User related issues for distribution
Feedback to users
Factors that can influence the delivery of plant material:
- Respond to requests with lists of material, forms and conditions of access and SMTA as soon as possible after receipt of request (users may not know about the conditions so it is better to inform them before proceeding with the request).
- Provide passport and germination data with requests.
Recommended procedures that ensure the material distributed matches the client request:
- On specific requests, match accession numbers with specified request.
-
If accession numbers are not specified, match accessions to users needs. These could include:
- Species.
- Plant habit to fit the crop system.
- Environment.
- Specific traits required, such as disease resistance.
- Use (e.g. consumer-related traits).
Feedback from users
The most relevant information required to be received from users:
- Information on characterization and evaluation/use (information on performance in one area allows better selection of germplasm for similar areas).
Quantity of material recommended to be distributed
Cassava accessions typically consist of a single clone, i.e there is no genetic variation within an accession. A single plant can represent the full diversity of the accession. The main consideration on quantity of material to send is to assure that the recipient has a very high probability to successfully recover the accession from in vitro culture.
- Three to five seedlings per accession are normally adequate.
Check availability
Availability in stock
- Check availability of vegetative material in the field or laboratory.
- Distribution of the requested material should not cause the accession to fall below the minimum stock or distribution of vegetative cuttings cause plant death.
- Accessions with little vegetative material or those which are not healthy should not be distributed.
Checking passport data
- Passport and evaluation data can be checked to ensure that the accession meets the requestor’s needs (this avoids waste of germplasm by sending material that the requestor does not need or want).
Preparing vegetative material for distribution
- Register the request: track requests according to the dates they are received (this allows requests to be handled on a first come first served basis).
- Prepare a list of accessions available: generate lists of accessions that meet users' needs.
- Check requirements for Standard Material Transfer Agreements (SMTA).
- Micropropagate or multiply the necessary plant material.
- Transfer material into multiplication or rooting media to promote the aerial growth and roots, to facilitate acclimatization.
- Prepare shipment, after 4-6 weeks of growth.
- Check the vigour of the plants before packaging. Only seedlings showing well-developed root and shoot systems should be considered for distribution.
- Pack the tubes carefully in boxes, with all the necessary labels. Use labels with good adhesive and clear printing (this avoids errors and mixing during shipping).
- Acquire the Phytosanitary Certificate to include in the shipment.
- Acquire the Import Permit.
-
Elaborate list of information to include in the shipment:
- Accession number.
- Accession identification.
- Crop name.
- Taxonomic identification.
- Country of origin.
- Biological status.
- Collecting location.
- Source.
- Characterization data used to verify accessions should be provided upon request.
- Cover letter, reminding users of the terms and conditions of access and requesting feedback (important to make contact with the user for future feedback).
- Include a handbook of procedures, explaining how the material should be handled. In vitro seedlings need special attention prior to transferring to field conditions.
- Register information into the plant genetic resources database.
|
|
Dispatching the plant material
- Pack the plant material for secure shipment,and to avoid damage or mixing the material.
- Attach documents e.g. the seed list, SMTA, Phytosanitary Certificate, Import Permit, GMO-free certificate in a strong envelope or a cardboard box.
- Attach a copy of the Phytosanitary Certificate, Import Permit and list of material to the outside of the box, if the material is being dispatched to another country.
- A copy of the SMTA must be attached to the outside of the envelope or box. Use of the material constitutes agreement with the terms of the SMTA.
- Label the envelope/box with the complete mailing address of the requester.
- Include a reply form (a reply form should be used to acknowledge that the material has been received by requester in good condition).
- Use courier or other rapid means of transit (method should avoid heating and delays in transport).
- Record date and method of shipping (shipping details are important for tracking during shipping).
- Update the genebank inventory.
Recording information during distribution
System for tracking material/inventory system for distribution
- Update related data tables in the database management system (a database system allows easy and fast access to data and allows macros to be written for routine operations).
- Use labels with good adhesive and clear printing (this avoids errors and mixing during shipping).
References and further reading
Ceballos H. 2006. Cassava research at CIAT [poster]. Centro Internacional de Agricultura Tropical (CIAT), Cali, Colombia. Available from: http://webapp.ciat.cgiar.org/news/pdf/poster02_scmeeting_06.pdf. Date accessed: 26 August 2010.
Guevara CL, Mafla G. 1996. Manihot collections held at CIAT. In: Engelmann F, editor. Management of field and in vitro Germplasm collections. CIAT, Cali, Colombia. pp. 109-112.
Mafla G, Roa JC, Debouck DG. 2004. Observations about the distribution of cassava germplasm from an international collection. Poster presented at the CBN-V. Available from: http://isa.ciat.cgiar.org/urg/urgweb_folder/files/posters/CBN-V.%20G%20Mafla%20ppt.pdf. Date accessed: 30 August 2010.
Mafla G, Roa JC, Flor NC, Aranzales E, Debouck DG. 2006. Distribution of cassava germplasm from an international genebank: a service to the global agriculture. Poster presented at the first meeting of the Governing Body, ITPGRFA, Madrid, Spain, 12-16 June 2006. Available from: http://ciat-library.ciat.cgiar.org/Articulos_Ciat/CIAT40years.pdf. Date accessed: 30 August 2010.
Viability and monitoring in cassava field banks
Contributors to this page: IITA, Nigeria (Dominique Dumet), Bioversity International/ILRI, Ethiopia (Alexandra Jorge); INIA, Peru (Llerme Rios); independent consultant (Clair Hershey).
Health diagnosis in field banks
- Preferably, test the source plants to identify healthy plants as a source of cuttings.
- If possible, do random health tests during field monitoring and checks.
- Follow recommendations from the cassava health diagnosis menu for detailed procedures.
Routine monitoring methods
Assures the viability, adequate growth and good health of the plants during field growth.
- Secure and protect the field against theft, vandalism and damage by animals.
- Weekly or bi-weekly (maximum) surveying of the collection is essential, to be aware of any problems that arise and need to be corrected.
- Check regularly for plant vigour and survival rates.
- Check regularly for mixtures due to handling mistakes: look for uniform characteristics, such as colours of young apical leaves (expanded and non-expanded), petiole, cortex and external stem as well as pulp, cortex and external root colours; flowering and branching types, pubescence of young leaves, shape of the central lobe, internode length (leaf scars), storage root peduncle and surface texture.
- Check regularly for pests and diseases. Contact plant health experts to identify symptoms and recommended appropriate control measures.
- Weed the field regularly.
Recording information during routine monitoring in field banks
The following information should be recorded for each step:
- Site name and map/GPS reference.
- Name of collaborator.
- Field bank site name (a code to identify the site location).
- Plot reference (the plot number at the field site).
- Accession number; population identification.
- Date of monitoring (date when data is collected).
- Date of test (the date that the test was carried out).
- Results of tests and action taken (removal of plant or application of insecticide or quarantine measures).
- Name of staff (name of staff recording the data).
- Survival rates (number of plants alive).
- Details of plants removed (due to type mixtures or pests or diseases contamination).
- Damage [a score of 1-5 (where 5 is most damaged) or qualitative assessment of damage (insect, disease etc)].
- Vigour [assessment of vigour of the plants on a scale of 1-5 (where 5 is high)].
- Field management details (watering, fertilizer, weeding, pest and disease control, stresses recorded, others).
References and further reading
Fukuda WMG. 1996. Banco de germoplasma de mandioca: manejo, conservação e caracterização. Cruz das Almas, BA: EMBRAPA-CNPMF. 103p. (EMBRAPA-CNPMF, Documento, 68).
Hershey C. 2008. A Global conservation strategy for cassava (Manihot esculenta) and wild Manihot species. A consultancy report to CIAT, on behalf of the Global Crop Diversity Trust, Rome. (Final report under review).
IITA Genebank Manual Series, Cassava field bank operations at the International Institute of Tropical Agriculture (IITA). International Institute of Tropical Agriculture, Ibadan, Nigeria.
Mohd SS, Rao VR, editors. 2001. Establishment and Management of Field Genebank, a Training Manual. IPGRI-APO, Serdang. 121 p. Available here.
Field management in cassava field banks
Contributors to this page: IITA, Nigeria (Dominique Dumet), Bioversity International/ILRI, Ethiopia (Alexandra Jorge); INIA, Peru (Llerme Rios); independent consultant (Clair Hershey).
|
Contents: |
Establishment
Choice of environment
- Cassava grows well between 30°N and 30°S in areas where annual rainfall is more than 750 mm a year, and mean daily temperatures are above 18°C (low to medium altitude (1500–2000 m) tropics, or low altitude subtropics).
- Well-aerated, loose and light sandy loam soil is recommended for cassava. It is sensitive to frost but tolerant of long dry periods, soils with low pH, high aluminium and low fertility.
- The area where material is maintained should be as free as possible of diseases and insect pests that could cause losses of material or create difficulties in the transfer of clean planting material to other sites.
- If water is available either through irrigation or well-distributed rainfall, the crop can be planted at any time of the year; preferably at the beginning of the warm season (growth slows during cool weather).
- In places where rainfall is seasonal and irrigation is not available, delay planting until rains are reliable.
Field preparation
- Cassava can be maintained in field plantings as a perennial plant, but periodic renewal every one or two years is desirable to avoid problems of excessive vegetative growth, cumulative disease and insect problems and to facilitate maintenance generally.
- Allow an overlapping period in the field of at least six months between the ‘old’ and the newly planted field to ensure that material that did not germinate can be replanted and provide a constant supply of planting material for research programmes.
- As a reference, approximately 0.3 ha is needed for the maintenance of 1000 accessions (0.4 ha when cuttings are planted for germplasm characterization purposes).
Traditional field methods
- Field plots should be uniform in fertility, with light textured, deep, well-drained soil and as free as possible from noxious weeds.
- Avoid stony, clay, shallow, hard or waterlogged soils, or manage them to correct the problems.
- In sandy soils, apply minimum tillage to conserve soil, organic matter and moisture and reduce soil erosion.
- In poorly drained soils, make ridges or mounds to reduce water logging.
Innovative method developed at CIAT
- In order to combine the benefits of lower space requirements with continual availability of planting material for experimental use, CIAT devised a slow-growth system based on restricting the root development in small planting pots (bonsai effect).
- Plants occupied only a small fraction of the space they would occupy if allowed unlimited growth in the field.
- Maintaining a cassava germplasm collection in containers has the potential advantages of space savings, better protection against pests, diseases and weather-related damage, and labour savings.
- Disadvantages can include difficulty in using plants as a source of planting material for field trials (generally small and weak stems), cost of infrastructure and cost of material.
Field planting
Plot size and spacing will depend on the size and purpose of the collection, land availability and demand for planting material.
Layout
- It may be beneficial for management purposes to group germplasm according to vigour, plant height, and branching habit, establishing at least three groups: high, intermediate and low vigour.
- Establish a distance of 2.2, 1.5 and 1.0 m between plots for high, intermediate and low vigour groups respectively, to minimize competition while making efficient use of land area.
- Space plants 1.0–1.5 m apart if evaluations are to be made simultaneously, or closer if the collection is solely for germplasm maintenance (0.75–1.0 m within the row and 1.0 m between rows) to minimize weed growth and land requirements.
- Maintain a minimum of five and an optimum of ten plants per accession to ensure adequate survival and supply of planting material.
Planting
- Plant the stakes directly in the ground (so that half or two-thirds of the stake is covered) or in soil ridges or mounds, vertically or at an angle, or even bury them horizontally about 5 cm below the soil surface. The local planting practice of experienced cassava growers in the area can also provide a good guide.
- Identify plots very carefully, putting a plastic tag in the first plant of the left hand row of the plot. Place an extra label on a plastic, metal or strong wood stake in front of the plot.
- Note that three to five extra cuttings are planted behind each peg as backup in case some of the main cuttings die.
- Draw a field map of the collection immediately after planting, with each accession located on the map, including both plot numbers and accession numbers.
- Replant missing plants one month after planting.
Weed management
- Ensure adequate control of weeds pre-emergence by ploughing and harrowing the soil or applying pre-emergence herbicides before planting, and post-emergence with herbicide applications, inter-row weeders or regular manual weeding.
- Weed as often as necessary to avoid competition between plants. Weeding may be required up to four times per season, depending on the environment.
- Critical times are during the initial four months or until leaves form a canopy and weed growth is suppressed.
Irrigation
- The soil must be moist at planting; otherwise irrigation is required.
- If irrigation is not available, it is important to plant the collection at the beginning of the wet season when rain is reliable.
Fertilization
- Fertilization is usually not required for the sole purpose of germplasm maintenance. However, add manure at land preparation (e.g. cow dung or poultry manure) if necessary.
- Apply NPK 15:15:15, depending on the soil analysis, about eight weeks after planting, around the plant, not touching the stems or leaves.
Pruning
One of the problems of longer-term maintenance in the field can be excessive growth of some accessions.
- It has been learned that periodic trimming back of these accessions often creates entry points for pests and diseases and is therefore counter-productive. Instead, follow the guidelines for plant spacing given above.
Rotation
- Re-plant the genebank on new land every regeneration cycle.
- Rotate with grass or leguminous crops to break the cycle of certain root pathogens and prevent land degradation.
Common pests and diseases
The Americas have the greatest diversity of cassava pests, followed by Africa and then Asia.
Under natural conditions, pests and pathogens are often kept under control by a combination of natural enemies, host plant resistance, and management practices.
In genebanks, these controls are often absent or reduced and pest and disease management can become a major challenge.
- Damage in Africa is often high due to the lack of natural predators of pests.
- Damage tends to be seasonal. Often insect and mite pests are more damaging in the dry season and diseases more damaging in the wet season.
- Consult the cassava health diagnosis menu for detailed list of pests and diseases and procedures.
Pest and disease control
- Weekly or bi-weekly (maximum) surveying of the collection is essential, to be aware of any problems that arise and need to be corrected.
- Select healthy planting material. Do not take cuttings from plants that had leaf chlorosis, shoot tip die-back, cankers, fungus patches or streaks on the stems.
- Treat cuttings with pesticides and fungicides before planting, and the plants during the growth stage when necessary.
- Rogue and burn diseased plants regularly during the growth season (if it does not compromise the survival of a specific accession).
- After harvest, destroy discarded stems and roots that have disease symptoms or pest contamination.
- Use natural enemies against cassava pests as much as possible. Complement by applying appropriate pesticides as necessary.
- Weed the field regularly.
- In the worst situation, cuttings can be replaced with the backup stems.
Insecticide treatment
- Insecticide or miticide treatments may be required to prevent widespread invasion of white flies, green mites, mealy bugs, termites, grasshoppers and other pests.
- To be effective, treatments need to be applied at an early stage of insect development (visible eggs or larvae).
- Pesticides should always be used according to label instructions.
- When working with pesticides, worker safety is always of utmost importance. Gloves, mask, (to cover the nose and mouth), safety goggles and rain boots are needed to give the sprayers full protection.
Herbicide treatment
- Herbicides are applied both pre-emergence immediately after planting and post-emergence at three, six, and nine months after planting.
- For pre-emergence weed control, it is advisable to spray on a moist soil and prior to a forecast rain if possible, to facilitate diffusion of the chemical into the soil, in contact with weed seeds.
- At the earlier development stages (up to three months), plants are short and tender. Extreme caution is advised during spraying to avoid chemical contact with young plants by using a guard fitted to the nozzle of the spraying equipment.
- This precaution is not as important when the plant matures, but efforts should always be made to spray only on weeds.
- Herbicides should always be used according to label instructions.
- When working with herbcides, worker safety is always of utmost importance. Gloves, mask, (to cover the nose and mouth), safety goggles and rain boots are needed to give the sprayers full protection.
Harvesting
- Harvest the stakes at the end of the growing season (this guide does not refer to any root or seed harvesting, dealing only with the vegetative propagules), usually 12–18 months after planting, depending on the cultivars and environment. In some environments most of the leaves will have dropped, but in others, a leaf canopy remains at maturity.
- Be careful to identify the stem cuttings from each plot.
Post-harvest management
- Store the stakes in a well-ventilated and shaded cool place until planting or in case they need to be replanted (keep extra planting material for a while until the collection is established).
- Take care during the harvesting and subsequent handling of the stakes not to bruise them.
- Extend storage time (not recommended for collections) with longer uncut stakes tied in bundles pre-treated in pesticide, at 70–80% RH and 20–23°C.
- Stakes can also be stored (also not recommended for collections) buried in the ground for several months, with the basal side down, or laid horizontally; regular watering is required to avoid excessive dehydration.
- Stakes or cuttings also store well for weeks in polythene bags in drier areas and/or during the dry season.
System for tracking material/inventory system during field bank storage
- Use pegs, tags or barcodes for labelling.
- Use impermeable ink and write clearly.
- Plots must be well labelled to avoid errors.
- Barcodes help avoid errors in recording.
Recording information during field bank storage
The following information should be recorded for each step:
- Site name and map/GPS reference.
- Name of collaborator.
- Field genebank site name (a code to identify the site location).
- Plot reference (the plot number at the field site).
- Accession number; population identification.
- Name of staff (name of staff recording the data).
- Method of planting, date and spacing.
- Field layout used.
- Field management details (watering, fertilizer, weeding, pest and disease control, stresses recorded, others).
- Environmental conditions (altitude, precipitation, temperature, soil type and others).
- Number of plants established.
- Days from planting to flowering (note: this will only be important if seed collection is anticipated).
- Harvest date and method.
- Number of plants harvested.
- Quantity of cuttings harvested.
- Comparisons with reference materials (record any identification numbers or references of any samples taken from the plots).
- Any evaluation undertaken during the growing period or at harvest.
- Post harvest (describe any relevant procedures).
- Others.
References and further reading
Fukuda WMG. 1996. Banco de germoplasma de mandioca: manejo, conservação e caracterização. Cruz das Almas, BA: EMBRAPA-CNPMF. 103 p. (EMBRAPA-CNPMF, Documento, 68).
Frison EA, Feliu E, editors. 1991. FAO/IBPGR Technical Guidelines for the Safe Movement of Cassava Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
IITA Genebank Manual Series, Cassava field bank operations at the International Institute of Tropical Agriculture (IITA). International Institute of Tropical Agriculture, Ibadan, Nigeria.
Mohd SS, Rao VR, editors. 2001. Establishment and Management of Field Genebank, a Training Manual. IPGRI-APO, Serdang. 121 p. Available here.
Sample preparation in cassava field banks
Contributors to this page: IITA, Nigeria (Dominique Dumet), Bioversity International/ILRI, Ethiopia (Alexandra Jorge); INIA, Peru (Llerme Rios); independent consultant (Clair Hershey).
Source of planting material
Cassava has a typical growing cycle between 9 and 24 months, depending on the genotype and the environmental conditions.
- It is best to regenerate within 18–24 months, when most plants complete their growth cycle, to avoid lodging from excessive growth and build-up of pests and diseases.
Visual inspection of plant material
- Inspect all plants in the plot to make certain they all appear to be the same clone. If there is a mixture of clones, reconfirm through the standard descriptors, by comparison with the previously recorded genebank descriptors, which of the plants are the true accession and which are the 'contaminants'.
- Select plants as sources of planting material based on their apparent health status. Plants should be free of virus symptoms and of other pests or diseases.
- Select plants that will provide well-lignified cuttings with well-distributed, healthy buds.
- Prepare stakes from healthy plants, identified earlier in the season before leaves drop off when pest and disease (especially virus) symptoms and other foliar diseases are apparent. Also inspect roots for pest and disease symptoms.
- Select the mature portion of the stem, avoiding the top green stems and the bottom section of the plants.
- Take care to avoid mixing of genotypes.
Preparation of planting material
- Cut stakes (stem pieces) at least 20 cm long with at least 4–5 nodes with viable buds to ensure crop establishment. Use well-lignified stems, generally from the middle section of the plant. Cut them at a right angle with a machete or saw to create a smooth cut.
- Handle stems with care to prevent bruising and peeling. Do not place the stems on a hard surface to cut them, as this can damage the nodes, reduce their quality and provide entry points for pathogens and insect pests.
- Tie cuttings of each accession firmly in separate bundles. At least one cutting in each bundle is labelled, and for extra security it is best to label two stakes (with accession name and number and date of harvest). The label must be sturdy and secure and able to withstand the handling (e.g. packaging and shipping) and treatment of the stake bundles.
Pre-treatments
- Treat the bundled stakes with a mixture of broad spectrum insecticide and fungicide.
- Add zinc sulphate in regions where zinc is limited in the soil.
Disposal of contaminated material
- Incinerate or autoclave contaminated material (to avoid spreading diseases and pests).
- Rogue and burn diseased plants regularly during the growth season (if it does not compromise the survival of a specific accession).
- After harvest, destroy discarded stems and roots that have disease symptoms or pest contamination
Recording information during sample preparation in field banks
The following information should be recorded for each step:
- Site name and map/GPS reference.
- Name of collaborator.
- Field bank site name (a code to identify the site location).
- Plot reference (the plot number at the field site).
- Accession number; population identification.
- Name of staff (name of staff recording the data).
- Source of cuttings.
- Number of generations since acquisition of germplasm or date of previous multiplication (if generation is not known).
- Preparation of planting material (details of pre-treatments applied).
- Details of plants removed or destroyed (due to type mixtures or pest or disease contamination).
References and further reading
Fukuda WMG. 1996. Banco de germoplasma de mandioca: manejo, conservação e caracterização. Cruz das Almas, BA: EMBRAPA-CNPMF. 103 p. (EMBRAPA-CNPMF, Documento, 68).
Hershey C. 2008. A Global conservation strategy for cassava (Manihot esculenta) and wild Manihot species. A consultancy report to CIAT, on behalf of the Global Crop Diversity Trust, Rome. (Final report under review).
IITA Genebank Manual Series, Cassava field bank operations at the International Institute of Tropical Agriculture (IITA). International Institute of Tropical Agriculture, Ibadan, Nigeria.
Mohd SS, Rao VR, editors. 2001. Establishment and Management of Field Genebank, a Training Manual. IPGRI-APO, Serdang. 121 p. Available here.
More Articles...
Subcategories
-
main
- Article Count:
- 1
-
Safety duplication
- Article Count:
- 1
-
Characterization
- Article Count:
- 1
-
Conservation
- Article Count:
- 11
-
Regeneration
- Article Count:
- 2