CGKB News and events Conservation
Conservation of cassava genetic resources
Contributors to this page: CIAT, Colombia (Daniel Debouck); IITA, Nigeria (Dominique Dumet); Bioversity International/ILRI, Ethiopia (Alexandra Jorge); independent consultant (Clair Hershey); INIA, Peru (Llerme Rios).
Importance of cassava conservation
The potential for genetic improvement of any crop relies on the ability to successfully use the existing genetic resources, including the related wild species. Cassava is the fourth most important food calorie crop in the tropics, and is growing in importance both for food security (especially Africa) and in multiple commercial and industrial uses (mainly Latin America and Asia). The current cassava genetic resources are primarily landrace varieties of Manihot esculenta and wild Manihot species that evolved under natural selection, as well as farmer conditions (cultivated cassava) for centuries or even millennia. In the only comprehensive monograph on the genus, Rogers and Appan (1973) identified 98 species in the genus. Most of the genetic diversification occurred in the Americas where the genus originated, but secondary centers of diversity also exist in Asia and Africa.
It is estimated that nearly 2/3 of the genetic diversity exists only in situ, with only 1/3 being securely conserved ex situ. However, these estimates are based on expert opinion rather than on solid survey evidence and genetic analyses (Hershey, 2008).
- There are still many unique native landrace varieties to be collected and safely conserved.
On a global basis, cassava has relatively few improved varieties relative to many other important crops, since there are very few fully-fledged breeding programmes in the world, and most of these operate with very constrained resources. Most cassava germplasm in use is still based on landrace varieties. However, the adoption of new improved varieties has increased rapidly since the turn of the 21st century. In some cases, this adoption of new varieties is creating a risk for loss of native landraces.
|
Figure 1 (Source: CIAT cassava programme) |
- Overall, farmers are adopting new varieties faster in Asia than elsewhere, especially in areas with high industrial demand. Landraces may be neglected and lost when adoption rates are high. Nonetheless, the areas where adoption rates are highest – China, Thailand and Vietnam – have very few landrace varieties and the development of new hybrids based on germplasm from the Americas is in fact introducing greater genetic diversity compared to what existed previously.
- In Africa, new varieties have been adopted due to increasing vulnerability of landraces to pests and diseases and higher productivity of new varieties. Landraces tend to disappear and many countries do not have adequate genebank conservation facilities.
- Expansion of agriculture and urbanization in South and Meso-America are shrinking the habitats of wild Manihot species.
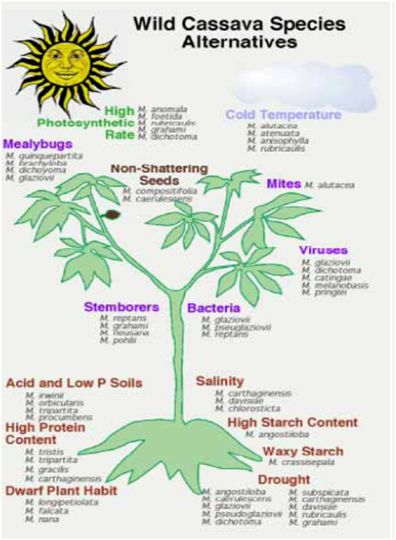
- Wild species have played a limited but important role to date in cassava breeding programmes. However, the new challenges of cassava breeding, especially considering the new traits that will be needed for adaptation to climate change, and for new markets, are creating demand for new gene sources. This emphasizes and supports the need to collect and conserve wild types. To date there has been limited evaluation of the wild species for traits that may be relevant to the future of cassava breeding. Major efforts are needed to collect and conserve the Manihot species, especially those in areas where urbanization and agricultural expansion are threatening native habitats, such as in central Brazil.
- Figure 1 illustrates examples of the many possible traits that might be derived from wild Manihot species. However, it should be noted that most of these traits are hypothetical, as proper evaluations have not yet been done for the most part.
Conservation methods
Cassava
The combinations of genes currently found in the cassava landraces are a result of many centuries of farmers’ selection. Since cassava is a highly heterozygous crop, the only way to preserve these specific gene combinations is through vegetative propagation. In all cases, when seeds are produced from a particular landrace accession, there is broad segregation of the progeny due to the heterozygous nature of the parents. If accessions are to be recommended and used directly as new varieties by farmers, then it is critical that these specific gene combinations are preserved, for the desired traits to be expressed. However, if accessions are to be used by breeders as sources of specific genes, then the specific gene combinations are less important. In this case, seed conservation may be as good as, or better than, clonal conservation.
- The most widely used method of cassava conservation is through vegetative methods.
- Germplasm can be maintained vegetatively in fields or screenhouses (field banks) or in vitro (slow growth tissue culture or cryopreservation).
- When the objective is solely to conserve the genes (but not specific combinations of genes), true seed can be used instead, as long as flowering occurs. Seeds can also be conserved at low temperature and humidity or with cryo techniques.
- Genes can also be maintained for further use in the form of DNA (DNA banks) or cryopreserved pollen.
Genebanks need to have clear priorities as to which type of germplasm should be collected, efficiently conserved and securely duplicated.
- Small genebanks may be able to effectively and economically conserve all material, including accessions introduced from other countries and bred material.
- Larger genebanks should prioritize the conservation of local landraces, especially if resources do not allow adequate conservation of the entire collection that includes introduced material that is simultaneously conserved in other genebanks.
- If core collections have been defined, these may be given the highest priority, especially in terms of making material available for evaluation and breeding. Core collections are typically relevant only for large collections and there are only a few of these worldwide.
- Conservation of breeding lines and introductions should typically have lower priority than local material.
The conservation of local landraces in particular must be secured. Additional security can be provided by the following procedures:
- Duplicate in vitro collections in separate locations (preferred).
- Maintain a field bank and an in vitro bank.
- Use two separate field locations (least preferred).
Wild species
The wild Manihot species are conserved mainly in seed genebanks or as plants in the field. Since they are perennials, field genebanks may be maintained for long periods without regeneration if conditions are appropriate. Generally, the wild Manihot genebanks are planted or regenerated using seed. Some of the species have been successfully conserved and propagated in vitro; however each species appears to have unique optimum media and environmental conditions, and the research to define the suitable conditions has been done for only a relatively few species.
Major cassava collections
The largest ex situ cassava collections are held by CIAT-Colombia with about 6500 accessions, followed by EMBRAPA-Brazil (about 4000 accessions). Other important genebanks are those in CTCRI-India , INIA-Peru, NRCRI-Nigeria, IITA-Nigeria, IAN-Paraguay, SRCV-Benin, D.R. Congo and PGRC/CRI-Ghana.
The exact number of unique accessions can only be estimated, since there has not been an attempt to identify duplicate accessions across genebanks. There are probably more than 10 000 unique accessions conserved ex situ, in the more than 70 cassava genebanks worldwide (Hershey, 2008). Although there are no scientifically- or survey-based estimates, Hershey (2008) estimated more than 26 000 unique cassava landrace varieties, meaning that there are more uncollected landrace varieties than there are accessions already conserved ex situ. Clearly not every distinct genotype needs to be collected in order to capture the range of genetic diversity of the species. Hershey (2008) estimated that about 14 000 accessions, appropriately selected for genetic diversity, need to be collected and conserved to assure the long-term accessibility to the full range of genetic diversity of Manihot esculenta, and avoid genetic erosion of the species.
Many genebanks have part of their collection in tissue culture (in vitro). Among the major collections, only the CIAT genebank conserves the the collection exclusively in tissue culture or in cryo conditions. A few genebanks (mainly EMBRAPA, Brazil and CIAT, Colombia) have seed banks to conserve seeds of wild species or breeding material. A few are also initiating DNA banks.
CIAT and IITA are the two CGIAR centres sharing the international mandate for cassava research, breeding and improvement. They have been disseminating thousands of in vitro plantlets of improved varieties to dozens of countries through collaborating NARs or the private sector and development agencies. In addition, a very wide range of genetic diversity has been introduced from the CGIAR genebanks into national programmes through seed introductions. However, this is typically done by breeding programmes and does not necessarily become part of the cassava genebank.
The wild Manihot species have been given relatively little attention in terms of systematic collection and ex situ conservation. Firstly, many populations of these species have not been sampled and, secondly, many collected accessions have been lost due to difficulty in establishing and maintaining them.
References and further reading
Allem AC. 1992. Manihot germplasm collecting priorities. In: Roca WM, Thro AM, editors. Report of the First meeting of the International Network for Cassava Genetic Resources, 18-23 August 1992. Centro Internacional de Agricultura Tropical, Cali, Colombia. pp. 87-110.
Bonierbale M, Guevara C, Dixon AGO, Ng NQ, Asiedu R, Ng SYC. 1997. Cassava. In: Fuccillo D, Sears L, Stapleton P, editors. Biodiversity in Trust. Cambridge University Press. pp. 1-21.
Debouck DG. 1991. Genetic variation in crop species and their wild relatives: a viewpoint for their conservation. In: Becker B, editor. Genetic diversity and crop strategies for roots and tubers. Arbeitsgemeinschaft Tropische und Subtropische Agrarforschung e.V. and International Board for Plant Genetic Resources, Bonn, Germany, pp. 41-51.
Epperson JE, Pachico DH, Guevara CL. 1996. The cost of maintaining genetic resources of cassava, Manihot esculenta Crantz. Acta Horticulturae 429:409-413.
Epperson JE, Pachico DH, Guevara CL. 1997. A cost analysis of maintaining cassava plant genetic resources. Crop Science 37(5): 1641-1649.
Guevara C, Ospina JA, Mafla G, Verdier V. 1998. Zygotic embryo culture of Manihot esculenta Crantz: a practical approach for the safe international movement of cassava seed stocks. FAO/Bioversity Plant Genetic Resources Newsletter 115:33-38.
Gulick P, Hershey C, Esquinas AJ. 1983. Genetic resources of cassava and wild relatives. Series AGPG:IBPGR/82/111. IBPGR Secretariat, Rome. 60 pp.
Hershey C. 2008. A global conservation strategy for cassava (Manihot esculenta) and wild Manihot species. Summary of stakeholder deliberations and recommendations prepared for the Global Crop Diversity Trust. Available from: http://isa.ciat.cgiar.org/urg/urgweb_folder/files/unitfiles/A%20Global%20Conservation%20Strategy%20for%20Manihot%20%20August%202008-2.pdf. Date accessed: 7 Oct. 2010.
Hershey C, Iglesias C, Iwanaga M, Tohme J. 1994. Definition of a core collection for cassava. In International Network for Cassava Genetic Resources. Report of the First Meeting, CIAT, Cali, Colombia, 18-23 August 1992. International Crop Network Series No. 10, IPGRI, Rome, Italy. pp. 145-156.
Hidalgo R. 1995. Conservación Ex-Situ. In: "Memorias. Curso de Documentación de Recursos Fitogenéticos". Auspiciado por Universidad Nacional de Colombia, Bioversity y CIAT. Palmira. pp. 33-41.
Koo B, Pardey PG, Debouck D. 2004. CIAT genebank. In: Koo B, Pardey PG, Wright BD. Saving seeds: the economics of conserving crop genetic resources ex situ in the future harvest centres of the CGIAR. CABI Publishing, Wallingford, UK.
Leyton M. 1993. Crioconservación de pollen de yucca. Bachelor’s thesis. Univ. del Valle, Facultad de Ciencias, Dept. de Biología, Cali, Colombia.
Nassar NMA. 2006. Cassava genetic resources: extinct everywhere in Brazil. Genetic Resources and Crop Evolution 53:975-983.
Orrego JI, Hershey C. 1984. Almacenamieno del polen de yuca (Manihot esculenta Crantz) por medio de liofilizacion y varios regimenes de humedad y temperatura. [Storage of cassava (Manihot esculenta Crantz) pollen by means of lyophilization and various temperature and humidity regimes]. Acta Agronomica (Colombia) 34:21-25.
Patiño VM, Hershey CH. 1981. Collection priorities for cassava (Manihot esculenta) and wild Manihot species in Latin America. Series AGPG:IBPGR/81/95. IBPGR, Rome, Italy.
Perry M. 1994. Documentation and databases for germplasm collections. In International Network for Cassava Genetic Resources. Report of the First meeting, CIAT, Cali, Colombia, 18-23 August 1992. International Crop Network Series No. 10, IPGRI, Rome, Italy. pp. 157-162.
Rogers DJ, Appan SG. 1973. Manihot, Manihotoides (Euphorbiaceae), Flora Neotropica, Monograph no. 13. Hafner Press, New York. 272 pp.
Unnikrishnan M, Easwari Amma CS, Sreekumari MT, Sheela MN, Mohan C. 1987. Cassava germplasm conservation and improvement in India. In Proceedings of a cassava workshop in Asia. February 1987. Available from: http://webapp.ciat.cgiar.org/asia_cassava/pdf/proceedings_workshop_02/87.pdf. Date accessed: 26 August 2010.
In vitro conservation of cassava genetic resources
Contributors to this page: CIAT, Colombia (Daniel Debouck, Roosevelt Escobar, Graciela Mafla); IITA, Nigeria (Dominique Dumet, Badara Gueye); Bioversity International, France (Ines Van den Houwe, Bart Panis, Nicolas Roux); Bioversity International/ILRI, Ethiopia (Alexandra Jorge); INIA, Peru (Llerme Rios); independent consultants (Erica Benson, Keith Harding, Clair Hershey).
What is in vitro conservation
|
|
In vitro techniques have been increasingly used for plants in the last 40-50 years. Conservation of genebank accessions is only one of several uses for the techniques. The basic procedure consists of conserving parts of plants in flasks or tubes in artificial media, under controlled environments, normally in sterile conditions. A prerequisite of successful in vitro conservation is that the plants or plant parts can be regenerated into complete plants for growth and use in any of the ways that genetic resources are used. Conservation of cassava in vitro is normally under slow growth storage (SGS), in conditions of controlled light and temperature and growth retardant chemicals.
While cryopreservation for long-term storage (LTS) is also technically an in vitro technique, the procedures are quite different from slow growth in vitro procedures, and they are discussed in a separate section.
Where is it used
An increasing number of countries has invested in tissue culture laboratories since the 1980s, when the techniques became broadly applicable for the propagation of clonal crops, including cassava. International collections are held at CIAT and at IITA, while all other in vitro genebanks have a national or regional focus. Many of these laboratories combine goals for in vitro techniques for pathogen cleaning, rapid multiplication and gene bank conservation.
Ng and Ng (2002) reviewed the status of in vitro cassava genebanks around the world. While they noted 47 countries with genebanks, only 12 countries maintained in vitro genebanks. Since there has not been a comprehensive survey of cassava genebanks, this may not be a complete listing. Nonetheless, it does appear to indicate that in vitro conservation of cassava is still far less common than field conservation. The largest national in vitro collections are held in Brazil and Argentina. There appear to be very few in vitro genebanks in Africa.
When it should be used
In vitro techniques for cassava conservation should be used whenever technical expertise, facilities and operating budget are available. They are generally more economical and less risky in a long-term perspective, as compared to field collections. Usually activities initiate with the use of traditional tissue culture techniques, mostly for the elimination of pests and diseases (also using meristem and thermotherapy techniques) and for the exchange and dissemination of germplasm. For conservation purposes, slow growth conditions are the only appropriate method and these are the focus of this section. Very few genebanks have initiated cassava conservation techniques using cryopreservation techniques. CIAT is one of the pioneers in this new field of in vitro conservation.
In vitro laboratory, MARDI, Malaysia (photo: C Hershey)
In vitro techniques are used:
- To conserve vegetative plant parts of cassava germplasm.
- To conserve plantlets that result from embryo rescue of seeds, which may be especially useful for the wild Manihot species whose seeds are difficult to germinate.
- As a viable alternative to complement and reduce the large size required for field banks.
- To reduce space requirements and eliminate the possibility of losses due to edaphic and climatic factors, two of the major sources of losses in field genebanks.
- To duplicate field banks.
- To replace field banks.
- To allow national and international germplasm exchange.
- To assure more secure conservation of germplasm for future generations.
Costs (qualified labour, energy, supplies and infrastructure) are highly location-specific and economies of scale should be considered before the adoption of in vitro conservation.
If there are other functions that can be shared by the cassava in vitro laboratory and genebank, there will be considerable savings through more efficient use of space, labour, supplies and management. For example, the facilities could be used to conserve another crop (sweet potato, banana, potato, etc.) or for cleaning and multiplying new varieties for distribution after release.
How should it be done
- It requires specialized laboratories and equipment with very skilled technicians and researchers.
- It also requires adaptive technologies for some more difficult species.
- It requires sterile conditions and very well controlled artificial growth environments.
- It requires high initial investments but relatively low maintenance costs in a long-term perspective.
- In vitro conservation can be more efficient and cost-effective if the laboratory forms part of a conservation strategy also involving other crops.
- In vitro conservation may be more efficient and cost-effective if the laboratory forms part of a system that also works with in vitro techniques of pest or pathogen cleaning and rapid multiplication technique.
References and further reading
Calles T, Dulloo ME, Engels JMM, Van den Houwe I. 2003. Best Practices for Germplasm Management - A new approach for achieving genebank standards. Technical Report. International Plant Genetic Resources Institute, Global Crop Diversity Trust, Rome, Italy.
Cuervo MI, Balcazar MS, Ramirez JL, Medina CA, Debouck D. 2009. Manual de operaciones laboratorio sanidad de germoplasma – unidad de recursos genéticos. GRP, CIAT, Colômbia. 72 pp. Available in Spanish (2.8 MB) and English (2.6 MB).
Escobar RM, Roca WM, Mafla G, Roa J. 1994. In vitro conservation of genetic resources: the case of cassava. CIAT (Internal Circulation). 23 p.
Escobar R, Mafla G, Roca WM. 1993. Cryopreservation of cassava shoot tips. In: Roca WM, Thro AM, editors. Proceedings First Scientific meeting of the Cassava Biotechnology Network, 25-28 Aug. 1992, Cartagena. CIAT, Cali, Colombia. pp. 116-121.
Fregene M, Ospina JA, Roca W. 1999. Recovery of cassava (Manihot esculenta Crantz) plants from culture of immature zygotic embryos. Plant Cell Reports 55:39-43.
IITA. 2007. Cassava in vitro processing and gene banking. IITA Genebank series 2007. Available here .
Mafla G, Roa JC, Guevara CL. 2000. Advances on the in vitro growth control of cassava using silver nitrate. In: Carvalho LJCB, Thro AM, Vilarinhos AD, editors. Proceedings IV International Scientific meeting of the Cassava Biotechnology Network, Salvador, Bahia, Brazil. November 03-07, 1998. EMBRAPA , CENARGEN and CBN. Brasilia, Brazil. pp. 439-446.
Mafla G. 1994. Conservación de germoplasma in vitro. In: King C, Osorio J, Salazar L, editors. Memorias I Seminario Nacional sobre Biotecnología. Universidad del Tolima. Colombia. pp 65-77.
Mafla G. 1995. Manejo de datos e información de la colección in vitro de yuca (Manihot esculenta Crantz). In: Memorias. Curso en Documentación de Recursos Fitogenéticos. Auspiciado por Universidad Nacional de Colombia, Bioversity y CIAT. Palmira, pp. 97-118.
Mafla G, Roa JC, Aranzales E, Debouck D. 2009. Handbook of procedures for in vitro germplasm conservation of the genus Manihot. CIAT, Cali, Colombia. 56 pp. Available here (8 MB).
Mafla G, Roa JC, Flor NC, Debouck DG. 2002. Conservación in vitro y utilización del germoplasma del género Manihot. Trabajo presentado en el VIII Congreso Latinoamericano de Botánica y II Congreso Colombiano de Botánica, Cartagena, Colombia, 13-18 Octubre 2002. Available from: http://isa.ciat.cgiar.org/urg/urgweb_folder/files/posters/cartagenafinal.pdf. Date accessed: 26 August 2010.
Mafla G, Roa JC, Ocampo C, Gallego G, Jaramillo G, Debouck DG. 2004. Efficacy of silver nitrate for slow growth conservation of cassava (Manihot esculenta Crantz). Determination of viability and genetic stability. In: Abstracts of the Sixth International Scientific meeting of the Cassava Biotechnology Network. March 8-14 CIAT, Cali, Colombia. p. 134.
Mafla G, Roa JC, Ocampo CH, Gallego G, Jaramillo G, Debouck DG. 2004. Efficacy of silver nitrate for slow-growth conservation of cassava (Manihot esculenta Crantz). Determination of viability and genetic stability. Poster presented at CBN-IV. Available from: http://isa.ciat.cgiar.org/urg/urgweb_folder/files/posters/CBN-VI.pdf. Date accessed: 26 August 2010.
Mafla G, Roa JC, Roca WM. 1990. Micropropagación de la yuca para la producción de material de siembra libre de enfermedades. In: Jaramillo J, y Agudelo O, editors. La nueva Biotecnología. Fundamentos, usos y perspectivas. ICA. pp. 91-101.
Mafla G, Roca WM, Reyes R, Roa JC, Muñoz L, Baca AE, Iwanaga M. 1992. In vitro management of cassava germplasm at CIAT. In: Roca WM, Thro AM, editors. Proceedings of First International Scientific meeting of the Cassava Biotechnology Network. Cartagena, Colombia. pp. 168-174.
Ng, NQ, Ng SYC. 2002. Genetic resources and conservation. In: Hillocks RJ, Thresh JM, Bellotti A, editors. Cassava: Biology, Production and Utilization. CABI Publishing. pp. 167-177.
Roca WM, Angel F, Sarria R, Mafla G. 1992. Future initiatives in biotechnology research for tropical agriculture: the case of cassava. In: McCorwick DK, editor. Advances in Gene Technology: Feeding the World in the 21st Century, 1992 Miami Bio/technology Winter Symposium, Miami, FL, USA, pp. 87.
Roca WM, Chaves R, Marin ML, Arias DI, Mafla G, Reyes R. 1989. In vitro methods of germplasm conservation. Genome 31(2):813-817.
Roca WM, Escobar R, Angel F, Mafla G. 1991. Tissue culture methods for germplasm conservation: The case of cassava. In: Bardowell ME, editor. Tissue culture technology for improved farm production, Kingston, Jamaica. pp 47-55.
Roca WM, Mafla G, Segovia RJ. 1991. Costo mínimo de un laboratorio de cultivo de tejidos vegetales. In: Roca WM, Mroginski LA, editors. Cultivo de tejidos en la agricultura: Fundamentos y Aplicaciones, pp. 912-920.
Roca WM, Nolt B, Mafla G, Roa JC, Reyes R. 1991. Eliminación de virus y propagación de clones en la yuca (Manihot esculenta Crantz). In: Roca WM, Mroginski LA, editors. Cultivo de tejidos en la agricultura: Fundamentos y Aplicaciones. pp. 403-421.
Szabados L, Nuñez LM, Tello LM, Mafla G, Roa JC, Roca WM. 1991. Agentes gelanitizadores en el cultivo de tejidos. In: Roca WM, Mroginski LA, editors. Cultivo de tejidos en la agricultura: Fundamentos y Aplicaciones. pp. 79-93.
Velásquez E, Mafla G. 1999. Conservación in vitro: Una alternativa segura para preservar especies silvestres de Manihot spp. (Euphorbiaceae). In: II Congreso Nacional de Conservación de la Biodiversidad. Pontificia Universidad Javeriana, Bogotá 19-22 Octubre 1999. p 14.
Cryo bank for cassava genetic resources
Contributors to this page: CIAT, Colombia (Daniel Debouck, Roosevelt Escobar, Graciela Mafla); IITA, Nigeria (Dominique Dumet, Badara Gueye); Bioversity International, France (Ines Van den Houwe, Bart Panis, Nicolas Roux); Bioversity International/ILRI, Ethiopia (Alexandra Jorge); INIA, Peru (Llerme Rios); independent consultants (Erica Benson, Keith Harding, Clair Hershey).
Introduction
In the last 25 years, several new cryoconservation procedures like vitrification, encapsulation-dehydration, preculture-dehydration, and encapsulation/vitrification have been established, which are all based on vitrification. Vitrification can be defined as the transition of water directly from the liquid phase into an amorphous or “glassy” phase, whilst avoiding the formation of crystalline ice. Cryoconservation protocols based on vitrification techniques have been developed for different vegetatively propagated crops, including cassava (Sakai and Engelmann 2007).
Cryoconservation is the storage of germplasm at ultra low temperatures (-196oC) using cryogenic media, typically liquid nitrogen. This temperature effectively stops the biological activity of the plant cells. This technique stops the metabolism of the in vitro plant, eliminating the need to regularly rejuvenate the plant.
Cryoconservation techniques have been increasingly used for the Long Term Storage (LTS) of cassava, through the freezing of embryos or shoot tips. It is currently an additional tool to improve conservation of germplasm in genebanks. CIAT pioneered research on cassava cryoconservation in 1985, and has given varying degrees of research emphasis to improving the techniques since that time.
Cyoconservation is also used for other purposes, such as the cryoconservation of embryogenic tissues for genetic transformation and for botanic seeds.
Current situation
Research on cryoconservation was initiated in the past few years in more than 600 accessions of cassava germplasm conserved at CIAT genebank. The main objective was to:
- Overcome some of the limitations of the in vitro maintenance (labour-intensive subculturing, potential for fungal and bacterial contaminants and somaclonal variation).
- To ensure the safe long-term conservation of cassava genetic resources.
This is a relatively new technique for cassava, and still with limited funding. The routine application of cryoconservation protocols for cassava shoot tip conservation has been pioneered by the CIAT genebank (Escobar et al. 1997, Gonzalez-Arnao et al. 2008, Roca 1984) modifying and adapting techniques initially developed for other crops in other laboratories. Specific cassava protocols were developed testing and optimizing a range of different cryogenic and non-cryogenic parameters. The method relies on rapid freezing of highly proliferating meristem cultures precultured for 2-3 months on 4E medium (Roca 1984). IITA recently started to test this methodology and adapt it to their conditions.
Many genebanks such as those at CIP, CGN, IPK, and USDA have also been using cryoconservation techniques for other important clonal, horticultural and herbaceous crops.
CIAT is the principal institution working on cryoconservation in cassava. New protocols for encapsulation dehydration and quick-freezing have now been developed and validated with more than 43% of the entire cassava core collection. More than 82% of the accessions tested have recovery rates of more than 30%, the minimum required for cryoconservation. However, this is still not a sufficient overall recovery rate for the entire CIAT collection: 18% of the sampled accessions fell below the minimum acceptable recovery rate. Protocols are now being adjusted for wild relatives of cassava, species of which sometimes behave very poorly in vitro or even in the field, making their conservation troublesome. Plants have been recovered for M. esculenta ssp. flabellifolia, M. esculenta ssp. peruviana and M. carthaginensis.
Cryoconservation is also being used to support transformation of cassava. Developing friable embryogenic callus cell lines is time consuming, with the inherent risks of genetic instability and low plant recovery over time.
When it should be used
- To conserve germplasm that can have more than 30% recovery. Some genotypes have very low percentages of recovery. Accessions that have been successfully recovered from cryo-conservation include some of the most common cultivated cultivars (M. esculenta) and some wild species (M. esculenta ssp. flabellifolia, M. esculenta ssp. peruviana and M. carthaginensis).
- To conserve and/or complement the conservation of materials that are not often used, or to duplicate materials maintained in tissue culture conditions.
- This is the most secure and trouble free conservation method for clonal cassava germplasm, but requires highly specialized personnel and equipment.
How it should be done
The following procedures, based on the colligative cryoprotection controlled rate cooling protocol, summarize the optimized methodologies as reported by Escobar et al. (1997):
- Explant: shoot tips 2 mm in height.
- Pre-culture: in medium (C4) comprising 1M sorbitol, 0.117M (4%) sucrose, 0.1 M DMSO for 3 days in the dark at 26 - 28ºC.
- Cryoprotection with 1M sorbitol, 0.117M (4%) sucrose, 10% DMSO for 2h on ice
- Tissue dehydration on filter paper for 1h.
- Controlled rate programmable freezing (CryoMed 1010) starting from a 5ºC chamber temperature, a rate of -0.5ºC min-1 to -15ºC, and thereafter at a rate of -1ºC min-1 to -40 ºC.
- Immersion in LN.
- Thawing at 37ºC.
- Sequential transfer recovery (2-days each) on medium containing (1) 0.75M sucrose with 0.2% activated charcoal and (2) half-strength MS medium with 0.35M sucrose and 5.5.6 x 10-3 M inositol in the dark; and standard culture medium under a light intensity of 15 μEm-2 s-1.
- Evaluation of tissue viability and shoot growth after 1 month.
There are often different genotype-dependent recovery responses.
- Apply different protocols to the same set of accessions to identify the most suitable for each type.
- Optimize cryo-conservation protocols on a case-by-case basis on very recalcitrant types.
- It is important to identify which types have high, intermediate or low response recovery rates. Protocols may need to be modified and adapted for different types.
- CIAT classified their genotype response into 3 categories:
- High response group – 70% of shooting.
- Intermediate response group – 30-70% of shooting.
- Low response group – Less than 30% of shooting.
References and further reading
CIAT. 2001. Cryopreservation of Cassava Germplasm, Its Wild Relatives, and Cell Lines for Tissue Culture. Available from: http://webapp.ciat.cgiar.org/biotechnology/crops_cassava.htm#cryo Date accessed: 26 August 2010.
Danso KE, Ford-Lloyd BV. 2004. Cryopreservation of embryogenic calli of cassava using sucrose cryoprotection and air desiccation. Plant Cell Reports 22: 623-631.
Escobar RH, Mafla G, Roca WM. 1993. Cryopreservation of cassava shoot tips. In: Roca WM, Thro AM, editors. Proceedings First Scientific Meeting of the Cassava Biotechnology Network, 25-28 Aug. 1992, Cartagena. CIAT, Cali, Colombia, pp. 116-121.
Escobar RH, Mafla G, Roca WM. 1997. A methodology for recovering cassava plants from shoot tips maintained in liquid nitrogen. Plant Cell Reports 16: 474-478. Available here.
Escobar RH, Manrique N, Munoz L, Rios A, Debouck D, Tohme J. Cassava cryopreservation by rapid freezing methodology. CIAT, Cali, Colombia. Available from: http://isa.ciat.cgiar.org/urg/urgweb_folder/files/unitfiles/Cassava%20cryopreservation%20by%20rapid%20freezing%20methodology.pdf. Date accessed: 6 October 2010.
Gonzalez-Arnao MT, Panta A, Roca WM, Escobar RH, Engelmann F. 2007. Development and large scale application of cryopreservation techniques for shoot and somatic embryo cultures of tropical crops. Available from: http://www.springerlink.com/content/y42117245j364361/fulltext.pdf Date accessed: 26 August 2010.
Marin ML, Mafla G, Roca WM, Withers LA. 1990. Conservation of cassava (Manihot esculenta Crantz): The role of cryopreservation. In: Proceedings of the VIIth International Congress on plant tissue and cell culture, Amsterdam, The Netherlands, 24-29 June 1990, pp. 371.
Mafla G, Roca WM, Kartha K. 1987. Conservation of Cassava (Manihot esculenta Crantz) germplasm in vitro, IV: Evaluation of phenotypic estability of plants grown from cryo preserved shoot tip cultures. In: Angarita A, editor. Abstracts of International Congress of plant tissue culture tropical species. Bogotá, Colombia, pp 75.
Mycock DJ, Wesley-Smith J, Berjak P. 1995. Cryopreservation of somatic embryos of four species with and without cryoprotectant pre-treatment. Ann. Bot., 75: 331-336.
Roca WM. 1984. Cassava. In: Sharp WR, Evans DA, Ammirato RV, Yamada Y. editors. Handbook of plant cell culture: crop species, vol 2. MacMillan Publishers, New York, pp 269-301.
Szabados L, Hoyos R, Roca W. 1987. In vitro somatic embryogenesis and plant regeneration of cassava. Available for purchase online from: http://www.springerlink.com/content/r53x32l17281215h/ Date accessed: 26 August 2010.
Seed banks for cassava genetic resources
Contributors to this page: CIAT, Colombia (Daniel Debouck); IITA, Nigeria (Dominique Dumet); Bioversity International/ILRI, Ethiopia (Alexandra Jorge); independent consultant (Clair Hershey); INIA, Peru (Llerme Rios).
When seed banks are used
Commercial plantings of cassava are done from cuttings (vegetative propagation), but many cultivated varieties also produce botanic seeds. Seed propagation is an important feature of breeding programmes – the principal means of recombining traits from different genotypes. In addition, all wild species of cassava are seed-propagated in nature and mostly seed-propagated in genebanks.
|
|
Seed banks are used:
- To conserve genes from cultivated types of cassava that produce botanic seeds.
- To conserve genes of wild species, which are difficult to maintain in field collections outside their natural habitat or under in vitro conditions.
- To conserve the genes of improved varieties and breeding lines.
Germplasm maintained in seed form is principally useful as a source of genes for breeding work and not directly as a source of varieties for the farmers, as the seeds only preserve the genes and not the specific gene combinations that define each variety. A large part of cassava seed storage is done by the breeders in their work of crossing and genetic improvement and is not formally part of a genebank system.
Further studies should be done in the future to fully take advantage of the long-term potential benefits of seed conservation of cassava as a part of the overall package of genetic resources management.
How it should be done
Cassava
|
|
There are no standardized recommendations for the production of cassava seeds for conservation in genebanks. Because cassava landraces are all highly heterozygous, the seeds they produce will segregate widely. It is not possible to reproduce the parent type through seed. However, seeds can be used to conserve the full array of genes, if the appropriate pollination and sampling methods are used. A working group on cassava conservation strategies (Hershey, 2008) suggested that the best way to develop seed populations would be by selfing the plants within an accession. This would have the advantage that only the genes from that accession are passed on through the seed, as opposed to “mixing” or “dilution” that would be caused by crossing with other accessions (as, for example, would occur if open-pollinated seeds were collected). A disadvantage of selfing as a means of seed production is that cassava suffers inbreeding depression in most cases. The plants that result from selfing will generally be less vigorous than the parent and, in some cases, this loss of vigour can compromise the survival of plants in the field and their ability to reproduce either vegetatively or sexually.
There are as yet no standards developed for the number of seeds that should be produced for selfed accessions of cassava as a means of conservation. In general, the main constraints will be the ability of a particular clone to flower and produce seeds and the high labour and management costs of controlled seed production. There is a need for further research on methods to enhance flower and seed production in cassava, as well as the effects of selfing across a wide range of genotypes.
Cassava seeds are orthodox in their behaviour and therefore should be conserved under conventional conditions of low humidity and low temperatures (Ellis et al. 1981). IITA (1979) reported storing seeds for up to seven years without losing germination at 5oC and 60% relative humidity. There do not appear to be any genebanks that conserve seeds from accessions as a means of conserving genetic diversity of Manihot esculenta. Nonetheless, it is a viable option that needs further study. Seeds can also be preserved in liquid nitrogen for a longer preservation period (Marin et al. 1990, Mumford and Grout 1978). Seeds can be treated with an insecticide/fungicide and stored in foil packages, as with other orthodox species such as the major cereal grains. Currently, however, there do not appear to be any genebanks storing cassava accessions in this manner.
Wild species
|
|
The wild species are all propagated by seed in their native habitats and the genebanks that conserve wild species typically propagate them by seed rather than vegetatively. Species vary in their germination ability. Some species benefit from embryo rescue but others not. It is generally recommended to plant seeds in controlled conditions in containers and then transplant healthy seedlings to the field. Some seeds of each accession should be kept as a reserve in cold storage.
References and further reading
Ellis RH, Hong TD, Roberts EH. 1981. The influence of desiccation on cassava seed germination and longevity. Ann. Bot., 47:173-175.
Iglesias C, Hershey C, Calle H, Bolaños A. 1994. Propagating cassava (Manihot esculenta) by sexual seed. Expl. Agric. 30:283-290.
Lima MC, Velasquez H, Santos LG, Debouck DG. GRU Handbook of procedures - seed conservation - pre-drying. GRU, CIAT, Colombia. 10pp. Available from: http://isa.ciat.cgiar.org/urg/urgweb_folder/files/handbookprocedures/en/GRU%20HandbookProceduresPreDryingApril.pdf. Date accessed: 7 October 2010.
Marin ML, Mafla G, Roca WM, Withers LA. 1990. Cryopreservation of cassava zygotic embryos and whole seeds in liquid nitrogen. Cryo-letters 11:257-264.
Mumford PM, Grout BWW. 1978. Germination and liquid nitrogen storage of cassava seed. Ann. Bot. 42:255-257.
Nassar NMA, O’Hair SK. 1985. Variation among cassava clones in relation to seed germination. Indian J. Genetics 45(2):394-398.
More Articles...
- Field banks for cassava and wild Manihot species
- Distribution of cassava genetic resources
- Viability and monitoring in cassava field banks
- Field management in cassava field banks
- Sample preparation in cassava field banks
- Slow growth storage (SGS) of cassava genetic resources
- Health diagnosis of cassava genetic resources



 Conservation
Conservation