CGKB News and events stog-clonal
Viruses - potato
Contributors to this section: CIP, Lima, Peru (Carols Chuquillanqui, Segundo Fuentes, Ivan Manrique, Giovanna Muller, Willmer Pérez, Reinhard Simon, David Tay, Liliam Gutarra); CIP, Nairobi, Kenya (Ian Barker); FERA, UK (Derek Tomlinson, Julian Smith, David Galsworthy, James Woodhall).
Scientific name
Potato Yellow Vein Virus (PYVV)
Significance
EPPO A1 quarantine organism.
Symptoms
Foliage: Symptoms of primary infection appear 10 to 15 days after infection as a bright yellowing of small (tertiary) leaf veins. Sometimes, only a few yellow spots developed. As the plants grew, secondary veins become affected. Later, leaf laminae may also become yellow. The main (primary) veins are rarely affected and remained green until plants died (Wale, 2008; Salazar, 1998).
Secondary infections: Symptom development in secondarily infected plants begins in the same way as in primarily infected plants. Some plants derived from tubers of infected plants are asymptomatic and the progeny of these individuals may develop both symptomatic and asymptomatic plants (Wale, 2008; Salazar, 1998).
Tubers: Reduction in number and size however can be identical to produced from healthy plants.
Hosts
Solanum tuberosum (potato)
The primary natural host of PYVV is potato. The known experimental host range of PYVV only includes Lycopersicon esculentum cv. Rutgers and the weed species Tagetes sp., Catharanthus roseus, Polygonum nepalense and Polygonum sp. (Salazar, 1998).
Geographic distribution
Europe, South America
Biology and transmission
PYVV is transmitted by the whitefly Trialeurodes vaporariorum (Vega, 1970; Tamayo and Navarro, 1984). Moreover, PYVV is perpetuated in potato tuber seed and can be transmitted by graft-inoculation, but not mechanically or by contact (Salazar, 1998). The virus is also transmitted through infected potato tubers. The virus can also infect and survive in weeds that play an important role in virus spread and establishment.
Detection/indexing method in place at CIP
- At CIP, the virus is detected by PCR and NASH.
Treatment/control
- In seed certification schemes, no virus infections must be tolerated during the growing season. Stocks of in vitro cultures used for propagation should be from pathogen-free plants and maintained under conditions designed to prevent infection and contamination.
Procedure followed at CIP in case of positive test
- In CIP if pathogen is detected the imported germplasm must be cleaned by thermotherapy.
References of protocols at EPPO, NAPPO or other similar organization
EPPO/OEPP. 2006. Phytosanitary procedures. Post-entry quarantine for potato. PM3/21(2)
OEPP/EPPO. 2008. EPPO Standards PM 1/ 2 (17). EPPO A1 and A2 lists of pests recommended for regulation as quarantine pests.
References and further reading
CABI. 2007. Crop Protection Compendium [online] Available from URL: www.cabi.org/compendia/cpc/ Commonwealth Agricultural Bureau International (CABI), Wallingford, UK. Date accessed 07 May 2010
Hooker WJ (ed.). 1981. Compendium of potato diseases. American Phytopathological Society. St. Paul, Minnesota, USA. 125 pp.
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the safe movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Salazar LF, Müller G, Owens RA, Querci M, Zapata JL. 1998. Identification of potato yellow vein virus (PYVV). In: Proceedings of the 14th Triennial Conference of the European Association for Potato Research, 63-64 (Abstract).
Tamayo PJ, Navarro R. 1984. Aumenta la incidencia del virus del amarillamiento de venas de la papa en Antioquia. ASCOLFI Informa (Bogotá), 10(5):40–42.
Vega JG, 1970. Transmisión, purificación y caracterización del agente causal del "amarillamiento de venas" en papa. M. Sc. Thesis. Universidad Nacional e Instituto Colombiano Agropecuario (ICA), Bogotá, Colombia, 47 pp.
Wale S, Platt HW (Bud), Cattlin N. 2008. Diseases, pests and disorders of Potatoes. A color Handbook. Manson Publishing, London, UK.176 pp.
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the safe movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Scientific name
Andean Potato Mottle Virus (APMoV)
Significance
EPPO A1 quarantine organism.
Symptoms
Foliage: Yellow local lesions or blotches, mild or severe mottle some leaf malformation. Dwarfing of plants. Secondary symptoms are more severe, leaf malformation and stunting (Jeffries, 1998, EPPO, 2007). Symptoms vary widely with potato cultivar (Fribourg, 2007 )
Tubers: No tuber symptoms have been reported, but the virus may induce delayed emergence of sprouts (Fribourg, 2007).
Hosts
APMoV is found naturally in Solanum tuberosum (potato) but can infects Capsicum annuum (bell pepper), Capsicum chinense (habanero pepper), Capsicum frutescens (chilli), Solanum melongena (aubergine or eggplant), Datura stramonium (jimsonweed), Nicandra physalodes (apple of Peru) and Nicotiana rustica (wild tobacco) (CABI, 2007).
Geographic distribution
Asia, Central America, South America
Biology and transmission
APMoV is transmitted by Diabrotica spp. (Fribourg et al., 1977). An isolate from Capsicum was transmitted by Diabrotica balteata (Valverde et al., 1995). Several wild potato species are susceptible to the virus and may be important as natural reservoirs from which the vectors could carry the virus to potato fields (Fribourg et al., 1977). The virus can be transmitted by contact between plants and is not transmitted by true seed, but is carried by potato tubers (Fribourg et al., 1977, Jeffries, 1998). Vegetative parts including seedlings and micropropagated plants are liable to carry the virus in trade. Growing medium accompanying plants doesn’t carry virus propagules (CABI, 2007).
Detection/indexing method in place at CIP
- At CIP, the virus is detected by DAS-ELISA and host range.
Treatment/control
- In seed certification schemes, no virus infections must be tolerated during the growing season. Stocks of in vitro cultures used for propagation should be from pathogen-free plants and maintained under conditions designed to prevent infection and contamination.
Procedure followed at the center in case of positive test
- In CIP if pathogen is detected the imported germplasm must be cleaned by thermotherapy.
References of protocols at EPPO, NAPPO or other similar organization
CABI/EPPO. Data sheets on Quarantine pest: potato Andean mottle commovirus. Prepared by CABI and EPPO for the European Communities. 4 p.
NAPPO. 2007. NAPPO Regional Standard for Phytosanitary Measures (RSPM). RSPM No. 3. Requirements for importation of potatoes into a NAPPO Member Country. Ottawa, Ontario, Canada. 53 pp.
OEPP/EPPO. 2008. EPPO Standards PM 1/ 2 (17). EPPO A1 and A2 lists of pests recommended for regulation as quarantine pests.
References and further reading
CABI. 2007. Crop Protection Compendium [online] Available from URL: www.cabi.org/compendia/cpc/ Commonwealth Agricultural Bureau International (CABI), Wallingford, UK. Date accessed 07 May 2010
Fribourg C. 2007. Virus, viroides y mollicutes de las plantas cultivadas en el Perú. Universidad Nacional Agraria La Molina. Lima, Perú. 219 p.
Fribourg CE, Jones RAC, Koenig R. 1977. Andean potato mottle, a new member of the cowpea mosaic virus group. Phytopathology 67(8):969–974
OEPP/EPPO. 1984. Data sheets on quarantine organisms No. 128. Potato viruses (non-European). Bulletin OEPP/EPPO Bulletin 14, 11–22.
Valverde RA, Black LL, Dufresne DJ. 1995. A comovirus affecting tabasco pepper in central America. Plant Disease, 79(4):421–423
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the safe movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Scientific name
Andean Potato Latent Virus (APLV)
Significance
EPPO A1 quarantine organism.
Symptoms
Foliage: Primary infections are latent or induce mild chlorotic netting of minor veins. Secondary symptoms range from mild to severe mosaic with necrotic flecking, curling and leaf-tip necrosis. The symptoms vary depending on virus strain, potato cultivar and environmental conditions (Jones and Fribourg, 1978). Symptoms were not observed in Ulluco (Lizárraga et al., 1996)
Hosts
In natural conditions APLV infects potato (Solanum tuberosum) and Ulluco (Ullucus tuberosus). The host range includes Solanaceae, Chenopodiaceae and Amaranthaceae (CABI, 2007; Jeffries, 1998).
Geographic distribution
Asia, South America
Biology and transmission
The virus is readily transmitted within the field by contact but is transmitted with low efficiency by the potato flee beetle (Epitrix sp.) and also by true potato seed (Jones and Fribourg, 1977). Transmission of APLV from an infected plant to its tubers was erratic (Jones and Fribourg, 1978). Vegetative parts including seedlings and micropropagated plants are liable to carry the virus in trade (CABI, 2007).
Detection/indexing method in place at CIP
- At CIP, the virus is detected by DAS-ELISA and host range.
Treatment/control
- In seed certification schemes, no virus infections must be tolerated during the growing season. Stocks of in vitro cultures used for propagation should be from pathogen-free plants and maintained under conditions designed to prevent infection and contamination.
Procedure followed at the center in case of positive test
- In CIP if pathogen is detected the imported germplasm must be cleaned by thermotherapy.
References of protocols at EPPO, NAPPO or other similar organization
CABI/EPPO. Data sheets on Quarantine pest: potato Andean latent tymovirus. Prepared by CABI and EPPO for the European Communities. 4 p.
NAPPO. 2007. NAPPO Regional Standard for Phytosanitary Measures (RSPM). RSPM No. 3. Requirements for importation of potatoes into a NAPPO Member Country. Ottawa, Ontario, Canada. 53 pp.
OEPP/EPPO. 1984. Data sheets on quarantine organisms No. 128. Potato viruses (non-European). Bulletin OEPP/EPPO Bulletin 14, 11–22.
OEPP/EPPO. 2008. EPPO Standards PM 1/ 2 (17). EPPO A1 and A2 lists of pests recommended for regulation as quarantine pests.
References and further reading
CABI. 2007. Crop Protection Compendium [online] Available from URL: www.cabi.org/compendia/cpc/ Commonwealth Agricultural Bureau International (CABI), Wallingford, UK. Date accessed 07 May 2010
Jones RAC, Fribourg CE. 1978. Symptoms induced by Andean potato latent virus in wild and cultivated potatoes. Potato Research, 21(2):121–127
Jones RAC, Fribourg CE. 1977. Beetle, contact and potato true seed transmission of Andean potato latent virus. Annals of Applied Biology, 86(1):123–128.
Lizárraga C, Santa Cruz M, Jayasinghe U.1996. Detection of an isolate of Andean potato latent tymovirus in ulluco (Ullucus tuberosus Caldas). Plant Disease, 80(3):344.
Lizárraga C, Santa Cruz M, Marca JL, Salazar LF.1999. The importance of the viruses infecting Ullucus tuberosus Caldas in Peru. Fitopatología 34(1):22–28.
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the safe movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Scientific name
Potato Yellowing Virus (PYV)
Other scientific names
Potato yellowing alfamovirus
Andean potato yellowing virus
Virus SB-22
Significance
EPPO A1 quarantine organism.
Symptoms
Foliage: Main symptoms include yellowing and premature senescence. The symptoms vary depending on potato cultivar (Jeffries, 1998, Fuentes y Jayasinghe, 1993)
Tuber: Effect on yield is unknown. (Fribourg, 2007; Valkonen et al., 1992)
Geographic distribution
South America
Biology and transmission
PYV is transmitted semi-persistently by Myzus persicae, and through true seed of Physalis floridana, Solanum tuberosum and Capsicum annuum (Fuentes, 1992; Valkonen et al., 1992). Vegetative parts including seedlings and micropropagated plants are liable to carry the virus in trade (CABI, 2007).
Detection/indexing method in place at CIP
- At CIP, the virus is detected by DAS-ELISA and host range.
Treatment/control
- In seed certification schemes, no virus infections must be tolerated during the growing season. Stocks of in vitro cultures used for propagation should be from pathogen-free plants and maintained under conditions designed to prevent infection and contamination.
Procedure followed at the center in case of positive test
- In CIP if pathogen is detected the imported germplasm must be cleaned by thermotherapy.
References of protocols at EPPO, NAPPO or other similar organization
CABI/EPPO. Data sheets on Quarantine pest: potato yellowing alfamovirus. Prepared by CABI and EPPO for the European Communities. 3 p.
NAPPO. 2007. NAPPO Regional Standard for Phytosanitary Measures (RSPM). RSPM No. 3. Requirements for importation of potatoes into a NAPPO Member Country. Ottawa, Ontario, Canada. 53 pp.
OEPP/EPPO. 2008. EPPO Standards PM 1/ 2 (17). EPPO A1 and A2 lists of pests recommended for regulation as quarantine pests.
References and further reading
CABI. 2007. Crop Protection Compendium [online] Available from URL: www.cabi.org/compendia/cpc/ Commonwealth Agricultural Bureau International (CABI), Wallingford, UK. Date accessed 07 May 2010
Fribourg C. 2007. Virus, viroides y mollicutes de las plantas cultivadas en el Perú. Universidad Nacional Agraria La Molina. Lima, Perú. 219 p.
Fuentes S. Jayasinghe YU.1993. Amarillamiento de la papa, causado por un nuevo virus baciliforme. Fitopatologia 28(1):22–37
Fuentes S. 1992. Identificación, características y distribución de un virus baciliforme aislado de papa (Solanum tuberosum L.). Tesis M. Sc. Universidad Nacional Agraria La Molina, Lima, Perú 184 p.
OEPP/EPPO. 1984. Data sheets on quarantine organisms No. 128. Potato viruses (non-European). Bulletin OEPP/EPPO Bulletin 14, 11–22.
Valkonen, J.P.T.; Pehu, E. and K. Watanabe. 1992. Symptom expression and seed transmission of alfalfa mosaic virus and potato yellowing virus (SB-22) in Solanum brevidens and S. etuberosum. Potato Research 35(4):403-410.
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the safe movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Potato yellow dwarf nucleorhabdovirus
Scientific name
Potato Yellow Dwarf Virus (PYDV)
Significance
EPPO A1 quarantine organism.
Symptoms
Foliage: PYDV causes stunted growth, dwarfing, apical yellowing and necrosis. Death of the apex occurs and is followed by necrosis of the upper axillary buds. Leaflet margins roll upward while the longitudinal axis curves downward. Pith necrosis of stems is common (Jeffries, 1998, CABI, 2007).
Tubers: Reduced in size, deformed with surface cracking and internal spots. Cracked tissues are covered with a corky layer (CABI, 2007).
Hosts
PYDV infects naturally wild Solanaceae and Chrysanthemum leucathemum var. pinnatifidum (ox-eye daisy). Artificially PYDV has been transmitted to species in the families Apocynaceae, Fabaceae, Lamiaceae, Polygonaceae and Scrophulariaceae (OEPP/EPPO, 1980; CABI, 2007). PYDV has been reported on ornamental herbaceous plants Mirabilis jalapa, Nicoitiana alata, Tagetes erecta and Zimmia elegans (Lockhart,1989).
Geographic distribution
North America
Biology and transmission
PYDV is transmitted in a persistent manner. One strain has been reported by its transmission by Aceratogallia sanguinolenta and second strain by Agallia constricta. Both strains can be transmitted by Agallia quadripunctata (Jeffries, 1998). PYDV is carried by potato tubers, but not by true seed. In principle, PYDV could be carried by potato tubers in international trade but micropropagated plants are not liable to carry the virus (CABI, 2007).
Detection/indexing method in place at CIP
- At CIP, virus is detected DAS-ELISA
Treatment/control
- In seed certification schemes, no virus infections must be tolerated during the growing season. Stocks of in vitro cultures used for propagation should be from pathogen-free plants and maintained under conditions designed to prevent infection and contamination.
Procedure followed at the center in case of positive test
- In CIP if pathogen is detected the imported germplasm must be cleaned by thermotherapy.
References of protocols at EPPO, NAPPO or other similar organization
NAPPO. 2007. NAPPO Regional Standard for Phytosanitary Measures (RSPM). RSPM No. 3. Requirements for importation of potatoes into a NAPPO Member Country. Ottawa, Ontario, Canada. 53 pp.
OEPP/EPPO. 1980. Data sheets on quarantine organisms No. 30. Potato Yellow Dwarf Virus. Bulletin OEPP/EPPO Bulletin 10(1):41–46.
OEPP/EPPO. 2008. EPPO Standards PM 1/ 2 (17). EPPO A1 and A2 lists of pests recommended for regulation as quarantine pests.
References and further reading
CABI. 2007. Crop Protection Compendium [online] Available from URL: www.cabi.org/compendia/cpc/ Commonwealth Agricultural Bureau International (CABI), Wallingford, UK. Date accessed 07 May 2010
Lockhar BEL.1989. Recurrence of natively occurring potato yellow dwarf virus in Minnesota. Plant Disease, 73(4):321–323.
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the safe movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Scientific name
Potato Black Ringspot Virus (PBRSV)
Other scientific names
Calico disease of potato
Tobacco ringspot virus (Andean potato calico strain) (Fribourg, 1977)
Significance
EPPO A1 quarantine organism.
Symptoms
Foliage: The symptoms vary depending on potato cultivar and virus strain. Systematically PBRSV cause necrotic spots and ringspots (Jeffries, 1998) and sometimes systemic necrosis (Fribourg, 1983). Calico-like symptoms (bright yellow areas on the margins of middle and upper leaves) are caused in some potato cultivars. Most of the plant foliage may eventually turn yellow without stunting or leaf deformations (Fribourg, 1983).
Hosts
PBRSV infects naturally potatoes (Solanum tuberosum), Arracacia xanthorrhiza (arracacha) and Oxalis tuberosa (oca) (Salazar and Harrison, 1978; Lizarraga et al., 1994; Jeffries, 1998).
Geographic distribution
South America
Biology and transmission
Local spread is by contact between plants and possibly nematode vectors but none has been identified. PBRSV is readily transmitted through tubers (Salazar and Harrison, 1978) and through true seed (Jones, 1982). PBRSV could be also carried by tubers of oca or arracacha (CABI, 2007). Micropropagated plants are liable to carry the virus (CABI, 2007). PBRSV is serologically distantly related to Tobacco ringspot and Eucharis mottle viruses.
Detection / indexing method in place at CIP
- At CIP, virus detected by DAS-ELISA
Treatment/control
- In seed certification schemes, no virus infections must be tolerated during the growing season. Stocks of in vitro cultures used for propagation should be from pathogen-free plants and maintained under conditions designed to prevent infection and contamination.
Procedure followed at the center in case of positive test
- In CIP if pathogen is detected the imported germplasm must be cleaned by thermotherapy.
References of protocols at EPPO, NAPPO or other similar organization
CABI/EPPO. Data sheets on Quarantine pest: Potato black ringspot nepovirus. Prepared by CABI and EPPO for the European Communities. 3 p.
NAPPO, 2007. NAPPO Regional Standard for Phytosanitary Measures (RSPM). RSPM No. 3. Requirements for importation of potatoes into a NAPPO Member Country. Ottawa, Ontario, Canada. 53 pp.
OEPP/EPPO. 2008. EPPO Standards PM 1/ 2 (17). EPPO A1 and A2 lists of pests recommended for regulation as quarantine pests.
References and further reading
CABI. 2007. Crop Protection Compendium [online] Available from URL: www.cabi.org/compendia/cpc/ Commonwealth Agricultural Bureau International (CABI), Wallingford, UK. Date accessed 07 May 2010
Fribourg CE. 1977. Andean potato calico strain of tobacco ringspot virus. Phytopathology 67(2):174–178
Fribourg CE. 1983. Tobacco ringspot virus. In: Compendium of Potato Diseases (ed. By Hooker, W.J.), pp.84-85. American Phytopathological Society, St. Paul, Minnesota, USA
Jones RAC.1982. Tests for transmission of four potato viruses through potato true seed. Annals of Applied Biology 100(2):315–320
Lizarraga C, Chuquillanqui C, Jayasinghe U. 1994. A strain of PBRV (potato black rinspot virus) isolated from Arracacha (Arracacia xanthorhiza). Fitopatología 29:144–149.
Salazar LF, Harrison BD. 1978. Host range and properties of potato black ringspot virus. Annals of Applied Biology 90 (3): 375–386.
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the safe movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Scientific name
Potato Virus T (PVT)
Significance
EPPO A1 quarantine organism.
Symptoms
Symptomless in potato, occasionally causes necrosis or chlorotic symptoms in some potato cultivars (CABI, 2007, Jeffries, 1998). Experimentally potato clone CPC 2783 and cultivars King Edward and Cara show vein necrosis or systemic top necrosis (Fribourg, 2007)
Hosts
PVT infects naturally potatoes (Solanum tuberosum), Oca (Oxalis tuberosa), Ulluco (Ullucus tuberosus) and Mashua (Tropaeolum tuberosum) (Lizarraga et al., 2000, Jeffries, 1998). Some wild potatoes are infected experimentally but could be natural reservoirs (Fribourg, 2007).
Geographic distribution
South America
Biology and transmission
PVT is transmitted by potato tubers, true potato seed and pollen (Fribourg, 2007; CABI, 2007). Mechanical transmission (e.g., by machinery) included plant-to-plant is reported by Jones (1982). PVT could be also carried by tubers of oca, mashua or ulluco (CABI, 2007). Micropropagated plants are liable to carry the virus (CABI, 2007).
Detection/indexing method in place at CIP
- At CIP, the virus is detected by host range and NASH.
Treatment/control
- In seed certification schemes, no virus infections must be tolerated during the growing season. Stocks of in vitro cultures used for propagation should be from pathogen-free plants and maintained under conditions designed to prevent infection and contamination.
Procedure followed at the center in case of positive test
- In CIP if pathogen is detected the imported germplasm must be cleaned by thermotherapy.
References of protocols at EPPO, NAPPO or other similar organization
CABI/EPPO. Data sheets on Quarantine pest: Potato T Trichovirus. Prepared by CABI and EPPO for the European Communities. 3 p.
NAPPO, 2007. NAPPO Regional Standard for Phytosanitary Measures (RSPM). RSPM No. 3. Requirements for importation of potatoes into a NAPPO Member Country. Ottawa, Ontario, Canada. 53 pp.
References and further reading
CABI. 2007. Crop Protection Compendium [online] Available from URL: www.cabi.org/compendia/cpc/ Commonwealth Agricultural Bureau International (CABI), Wallingford, UK. Date accessed 07 May 2010
Fribourg C. 2007. Virus, viroides y mollicutes de las plantas cultivadas en el Perú. Universidad Nacional Agraria La Molina. Lima, Perú. 219 p.
Jones RAC. 1982. Tests for transmission of four potato viruses through potato true seed. Annals of Applied Biology 100(2):315–320
Lizarraga C, Querci M, Santa Cruz MS, Bartolini I, Salazar LF. 2000. Other natural hosts of Potato virus T. Plant disease 84 (7): 736–738
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the safe movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Scientific name
Potato Virus Y (PVY)
Strains on potato:
PVYc = stipple streak strain
PVYo =common strain
PVYN = tobacco veinal necrosis strain (including PVY NTN, the so-called tuber necrotic strain of N).
PVYZ = strain group
Significance
High importance. Yield losses reach 10-80%.
Symptoms
Foliage: Symptoms include mild and severe mosaic, rugosity; crinkling, dropping of leaves (leaf drop streak), severe systemic necrosis and dwarfing. Symptoms vary widely with virus strain and potato cultivar (Jeffries, 1998).
PVYN isolates usually cause only slight leaf symptoms in comparison to PVYo and PVYC, which cause much more severe symptoms (Jeffries, 1998).
Primary symptoms of PVYo and PVYC are necrosis, mottling, yellowing of leaflets, leaf drops and premature death of plants (Wale et al., 2008).
Plants with secondary PVYo infection are dwarfed and leaves are mottled and crinkled (Hooker, 1981).
Tubers: PVYNTN isolates cause severe superficial tuber necrosis (potato tuber necrotic ringspot disease) and may also cause necrotic foliar symptoms. Some PVYN isolates may also cause tuber necrosis (Jeffries, 1998).
Hosts
Peppers (Capsicum), bell pepper (Capsicum annuum), tomato (Lycopersicon esculentum), tobacco (Nicotiana tabacum), potato (Solanum tuberosum), nightshade (Solanum sp.), black nightshade (Solanum nigrum), tree tomato (Cyphomandra betacea), Dahlia and Petunia spp., Physalis spp.and Solanum dulcamara.
Geographic distribution
Asia, Europe, Africa, North America, Central America, South America, Oceania
Biology and transmission
PVY is transmitted in a non-persistent manner by more than 50 aphid species (Kennedy et al., 1962; Sigvald, 1984; Salazar, 1996; Ragsdale et al., 2001; Robert and Bourdin, 2001). Virus spread takes place mainly by winged aphids (Wale, 2008). PVY has not been reported in true seed but transmission can occur readily in potato tubers and other vegetative parts including seedlings and micropropagated plants. Growing medium accompanying plants doesn’t carry virus propagules (CABI, 2007). Incidence in seed potato tubers can be very high in the absence of certification schemes or with tolerant cultivars. Many plant species, mostly in the Solanaceae, but also in the Chenopodiaceae and Leguminosae are hosts to PVY. Potato volunteer plants are virus reservoir hosts (Hooker, 1981).
Detection/indexing method in place at CIP
- At CIP, the virus is detected by DAS-ELISA and host range.
Treatment/control
- In seed certification schemes, no virus infections must be tolerated during the growing season. Stocks of in vitro cultures used for propagation should be from pathogen-free plants and maintained under conditions designed to prevent infection and contamination.
Procedure followed at CIP in case of positive test
- In CIP if pathogen is detected the imported germplasm must be cleaned by thermotherapy.
References and further reading
CABI. 2007. Crop Protection Compendium [online] Available from URL: www.cabi.org/compendia/cpc/ Commonwealth Agricultural Bureau International (CABI), Wallingford, UK. Date accessed 07 May 2010
Hooker WJ. (ed.). 1981. Compendium of Potato Diseases. American Phytopathological Society. St. Paul, Minnesota, USA. 125 pp.
Jeffries C. 1998. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Kennedy JS, Day MF, Eastop VF.1962. A Conspectus of Aphids as Vectors of Plant Viruses. Wallingford, UK: CAB INTERNATIONAL.
Ragsdale DW, Radcliffe EB, DiFonzo CD. 2001. Epidemiology and field control of PVY and PLRV. In: Loebenstein G, Berger PH, Brunt AA, Lawson RH, eds. Virus and Virus-like Diseases of Potatoes and Production of Seed-Potatoes, pp. 237-270. Dordrecht, The Netherlands; Kluwer Academic Publishers.
Robert Y, Bourdin D. 2001. Aphid transmission of potato viruses. In: Loebenstein G, Berger PH, Brunt AA, Lawson RH, eds. Virus and Virus-like Diseases of Potatoes and Production of Seed-Potatoes, pp. 195-225. Dordrecht, The Netherlands; Kluwer Academic Publishers.
Salazar LF. 1996. Potato Viruses and their Control. Lima, Peru: International Potato Center, 214 pp.
Sigvald R. 1984. The relative efficiency of some aphid species as vectors of potato virus Y (PVY). Potato Research, 27(3):285-290.
Wale S, Platt HW (Bud), Cattlin N. 2008. Diseases, pests and disorders of Potatoes. A Color Handbook. Manson Publishing, London, UK.176 pp.
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Scientific name
Potato Virus X (PVX)
Significance
High importance.
Symptoms
Foliage: Mild mosaics and mottles. Symptom development, however, is dependent upon the interaction of cultivar, virus strain and environmental conditions. At higher temperatures than 25°C infection is asymptomatic. Other strains cause severe mosaic and leaf crinkling, or acute tip necrosis usually followed by plant death (Fribourg, 2007). Symptoms are especially severe when PVX is present together with other viruses.
Tubers: Tuber necrosis occurs in some cultivars (Jeffries, 1998).
 PVX (photo: CIP) |
Hosts
Bell pepper (Capsicum annuum), chili (Capsicum frutescens), tomato (Lycopersicon esculentum), tobacco (Nicotiana tabacum), potato (Solanum tuberosum), turnip (Brassica rapa subsp. rapa), globe artichoke (Cynara cardunculus L. var. scolymus), purple clover (Trifolium pratense), grapevine (Vitis vinifera), redroot pigweed (Amaranthus retrolexus), fat hen, lamb’s quarters (Chenopodium album), kangaroo apple (Solanum laciniatum)
Geographic distribution
Asia, Europe, Africa, North America, South America, Oceania
Biology and transmission
PVX is transmitted by contact from infected to healthy plant and also by farm machinery, clothes and animal skin presumably because it is very stable in vitro and highly infectious (Fribourg, 2007). It is also transmitted from infected to healthy sprouted tubers stored in the same bag (Bawden et al., 1948). Transmissions by zoospores of the fungus Synchytrium endobioticum (Nienhaus and Stille, 1965) have been reported. This is an interesting report as soil-borne transmission of PVX has been recorded (Salazar, 1996). Biting insects could occasionally transmit PVX by contact (Beemster, 1987).
Detection/indexing method in place at CIP
- Serotypes PVXo and PVXa , pathotypes 1 and HB (Fernandez-Northcote, 1990) and Strain-groups 1, 2, 3 and 4 (Cockerham 1955, 1970) have been reported in this virus.
- At CIP, the virus is detected by DAS-ELISA and host range.
Treatment/control
- In seed certification schemes, no virus infections must be tolerated during the growing season. Stocks of in vitro cultures used for propagation should be from pathogen-free plants and maintained under conditions designed to prevent infection and contamination.
Procedure followed at the centers in case of positive test
- In CIP if pathogen is detected the imported germplasm must be cleaned by thermotherapy.
References of protocols at EPPO, NAPPO or other similar organization
NAPPO. 2003. Regional standard for Phytosanitary Measures (RSPM) No.3. Requirements for importation of potatoes into a NAPPO member country. 53 pp.
OEPP/EPPO. 1998. EPPO certification scheme for seed potatoes. Bulletin OEPP/EPPO Bulletin 28:561-567.
References and further reading
Bawden FC, Kassanis B, Roberts FM.1948. Studies on the importance and control of potato virus X. Annals of Applied Biology, 35:250–265.
Beemster ABR, De Bokx JA. 1987. Survey of properties and symptoms. In: De Bokx JA, Van der Want JPH, eds. Viruses of potato and seed potato production. Wageningen, The Netherlands: Centre for Agricultural Publishing and Documentation, 84–113.
Cockerham G. 1955. Strains of potato virus X. Pp. 89-92 In: Proc. 2nd. Conf. Potato Virus diseases, Lisse-Wageningen, 1954.
Cockerham G. 1970. Genetical studies on resistance to potato viruses X and Y. Heredity 25:309-348.
Fernandez-Notrthcote EN. 1990. Variability of PVX and PVY and its relationship to genetic resistance. Pp. 131-139 In: International Potato Center (CIP). Control of Virus and Virus-like diseases of Potato and Sweet potato. Report of 3erd. Planning Conference, 20–22 Nov. 1989, Lima, Peru.
Fribourg C. 2007. Virus, viroides y mollicutes de las plantas cultivadas en el Perú. Universidad Nacional Agraria La Molina. Lima, Perú. 219 p.
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the safe movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Nienhaus F, Stille B.1965. Ubertragung des Kartoffel-X-Virus durch Zoosporen von Synchytrium endobioticum. Phytopathologische Zeitschrift, 54:335–337.
Salazar LF. 1996. Potato Viruses and their Control. Lima, Peru: International Potato Center, 214 pp.
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the safe movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Scientific name
Potato Virus S (PVS)
Two strain groups have been recognized, designated PVSo (ordinary) is distributed worldwide and PVSa (Andean) reported from Andean region of South America (Corzo, 1989; Hinostroza-Orihuela, 1973), and other parts of the world (Santillan, 1979; Cupertino y Oliveira, 1970; Slack, 1983; Rose, 1983).
Other scientific names
Potato S carlavirus
Pepino latent virus
Significance
Yield reduction ranges from 3 to 44% (Gladysiak and Wieckowski, 1977; Wright et al., 1977, Gandarillas, 1986; Corzo, 1989).
Symptoms
Symptoms are dependent mainly on incidence, virus strain, potato cultivar and environmental conditions. Generally, PVS is symptomless in many potato cultivars, however, some isolates cause undulation of leaf margins and some rugosity of leaf surfaces in susceptible potato cultivars and cause leaf bronzing, especially in very sensitive cultivars infected with virulent isolates (Beemster and de Bokx, 1987).
Hosts
Naturally restricted to potato and Pepino (Solanum muricatum).
Experimentally infects species of Chenopodiaceae and Solanaceae.
Geographic distribution
Asia, Europe, Africa, North America, South America, Oceania
Biology and transmission
PVS is readily transmitted mechanically (e.g., machinery) including plant-to-plant contact. Certain strains spread in a non-persistent manner by aphids, particularly Myzus persicae and Aphis nasturtti (Jeffries, 1998; Hooker, 1981). PVS has not been reported in true seed but transmission can occur readily in potato tubers and other vegetative parts including seedlings and micropropagated plants. Growing medium accompanying plants doesn’t carry virus propagules (CABI, 2007).
Detection/indexing method in place at CIP
- At CIP, the virus is detected by DAS-ELISA and host range.
Treatment/control
- In seed certification schemes, no virus infections must be tolerated during the growing season. Stocks of in vitro cultures used for propagation should be from pathogen-free plants and maintained under conditions designed to prevent infection and contamination.
Procedure followed at CIP in case of positive test
- In CIP if pathogen is detected the imported germplasm must be cleaned by thermotherapy.
References of protocols at EPPO, NAPPO or other similar organization
NAPPO. 2003. Regional standard for Phytosanitary Measures (RSPM) No.3. Requirements for importation of potatoes into a NAPPO member country. 53 pp.
OEPP/EPPO. 1998. EPPO certification scheme for seed potatoes. Bulletin OEPP/EPPO Bulletin 28:561–567.
References and further reading
Beemster ABR, de Bokx JA.1987. Survey of properties and symptoms. In: de Bokx, J.A. and van der Want, J.P.H. (Eds.) Viruses of Potato and Seed Potato Production. Wageningen, Netherlands: PUDOC, 84–113.
CABI. 2007. Crop Protection Compendium [online] Available from URL: www.cabi.org/compendia/cpc/ Commonwealth Agricultural Bureau International (CABI), Wallingford, UK. Date accessed 07 May 2010
Corzo P, Sánchez de Luque C, Malamud O, Salazar LF. 1989. Incidencia de virus en campos de producción de papa para consumo y para semilla. Fitopatología 24 (1):7–12
Cupertino FP, Oliveira AR. 1970. Presença do virus S em batata-semente nacional e estrangeira. Bragantia 29:17–20
Gandarillas A. 1986. Diseminación de algunos virus de papa en las zonas de Huancayo e Ica. Tesis M. Sci. Fitopatología. Universidad Nacional Agraria La Molina, Lima, Peru.
Gladysiak S, Wieckowski A.1977. Effect of secondary virus S infection on yield of two varieties of potato. Rocz. AR Pozn. No.97:89–94.
Hinostroza-Orihuela AM. 1973. Some properties of potato virus S isolated from Peruvian potatoes. Potato Research 16:244–250
Hooker WJ. (ed.). 1981. Compendium of Potato Diseases. American Phytopathological Society. St. Paul, Minnesota, USA. 125 pp.
Howell PJ. 1981. The risks from exotic potato viruses. EPPO Bulletin, 11(3):243–249.
Rose DG. 1983. Some properties of an unusual isolate of potato virus S. Potato Research 26:49–62.
Santillán F, Fribourg CE, Jones RAC. 1980. Estudio comparativo de 11 aislamientos de virus S de la papa (PVS) de la región andina. Fitopatología 15 (1):42–43.
Slack SA. 1983. Identification of an isolate of the Andean strain of potato virus S in North America. Plant. Dis. 67:786–789
Wright NS, Mellor FC, Cole EF, Hyams CM.1977. Control of PVX and PVS in seed potatoes. Canada Agriculture, 22(2):14–16;
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the safe movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Scientific name
Potato Virus M (PMV)
Significance
Often symptomless. Causes mottle, mosaic, crinkling and rolling of leaves (paracrinkle), and stunting of shoots. Symptoms mainly occur in plants infected at very young stage. Severity is influenced by virus isolate and potato cultivar.
Symptoms
Most strains of the virus induce no conspicuous symptoms in many potato cultivars (for example, Green Mountain, Irish Cobbler, Sebago and Up-to-Date). However, in some cultivars (for example, Climax, Fortuna, Hatahdin, King Edward and White Rose) some strains induce very mild leaf chlorosis and leaf distortion, and in a few (for example, cultivars Arran Victory) they can cause severe leaf mottling, chlorosis, crinkling and rolling and stunting. The severity of symptoms is dependent on the virulence of the infecting virus strain, the tolerance of the cultivar and, possibly, environmental factors (Beemster and Rozendaal, 1972).
Hosts
Potato is the major natural host of PVM.
Experimentally can be transmitted to another 122 species in 9 genera of the Solanaceae and to 47 species in the Amaranthaceae, Caryophyllaceae, Chenopodiaceae, Compositae, Cucurbitaceae, Fabaceae and Rubiaceae (Bagnall et al., 1956, 1959; Hiruki, 1970; de Bokx and Chrzanska, 1972; Kowalska and Was, 1976; Edwardson and Christie, 1997).
Geographic distribution
Asia, Europe, Africa, North America, South America.
Biology and transmission
The virus is transmitted in the non-persistent manner by aphids (MacKinnon, 1974). Some isolates, however may be transmitted mechanically (e.g., machinery) including plant-to-plant contact (Jeffries, 1998). The widespread geographical occurrence of the virus is probably attributable to the inadvertent international distribution of infected tubers. PVM has not been reported in true seed but transmission can occur readily in potato tubers and other vegetative parts including seedlings and micropropagated plants. Growing medium accompanying plants doesn’t carry virus propagules (CABI, 2007).
Detection/indexing method in place at CIP
- At CIP, virus is detected by DAS-ELISA
Treatment/control
- In seed certification schemes, no virus infections must be tolerated during the growing season. Stocks of in vitro cultures used for propagation should be from pathogen-free plants and maintained under conditions designed to prevent infection and contamination.
Procedure followed at CIP in case of positive test
- In CIP if pathogen is detected the imported germplasm must be cleaned by thermotherapy.
References of protocols at EPPO, NAPPO or other similar organization
NAPPO. 2003. Regional standard for Phytosanitary Measures (RSPM) No.3. Requirements for importation of potatoes into a NAPPO member country. 53 pp.
OEPP/EPPO. 1998. EPPO certification scheme for seed potatoes. Bulletin OEPP/EPPO Bulletin 28:561–567.
References and further reading
Bagnall RH, Larson RH, Walker JC.1956. Potato viruses M, S and X in relation to interveinal mosaic of the Irish Cobbler variety. Bulletin of the Wisconsin Agricultural Research Station No. 198.
Beemster ABR, Rozandaal A. 1972. Potato viruses; properties and symptoms. In: Bokx ,J.A. de (Ed.). Viruses of Potatoes and Seed-Potato Production. Pudoc, Wageningen, The Netherlands: PUDOC, 115–143.
CABI. 2007. Crop Protection Compendium [online] Available from URL: www.cabi.org/compendia/cpc/ Commonwealth Agricultural Bureau International (CABI), Wallingford, UK. Date accessed 07 May 2010
Edwardson JR, Christie RG. 1997. Viruses infecting peppers and other solanaceous crops; Volume 1:106–123. Gainesville, Florida, USA: University of Florida.
Hiruki C. 1970. Red kidney bean, a useful bioassay host for qualitative and quantitative work with potato virus M. Phytopathology, 60:739–740.
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the safe movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Kowalska A, Was M.1976. Detection of potato virus M and potato virus S on test plants. Potato Research, 19(2):131–139
MacKinnon JP. 1974. Detection, spread and aphid transmission of potato virus S. Canadian Journal of Botany, 52:461–465.
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the safe movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Scientific name
Potato Virus A (PVA)
Significance
Yield reduction can be up to 40% (Jeffries, 1998).
Symptoms
PVA causes mild mosaic. Mottles may appear on the veins, and infected leaves look shiny (Wale, 2008). Severe disease in combination with the potato virus x and / or PVY. Severity of symptom expression depends on whether conditions, potato cultivars and the strain of virus A (Hooker,1981)
Hosts
Mainly potato (Solanum tuberosum), peppers (Capsicum spp.) and tobacco (Nicotiana tabacum) (CABI, 2007)
Geographic distribution
Asia, Europe, Africa, North America, South America, Oceania
Biology and transmission
By aphids (Aphis frangulae, Macrosiphum euphorbiae and myzus persicae) in a non-persistent manner (Wale, 2008).
Detection/indexing method in place at CIP
- At CIP, virus is detected by DAS-ELISA
Treatment/control
- In seed certification schemes, no virus infections must be tolerated during the growing season. Stocks of in vitro cultures used for propagation should be from pathogen-free plants and maintained under conditions designed to prevent infection and contamination.
Procedure followed at CIP in case of positive test
- In CIP if pathogen is detected the imported germplasm must be cleaned by thermotherapy.
References of protocols at EPPO, NAPPO or other similar organization
NAPPO. 2003. Regional standard for Phytosanitary Measures (RSPM) No.3. Requirements for importation of potatoes into a NAPPO member country. 53 pp.
OEPP/EPPO. 1998. EPPO certification scheme for seed potatoes. Bulletin OEPP/EPPO Bulletin 28:561–567.
References and further reading
CABI. 2007. Crop Protection Compendium [online] Available from URL: www.cabi.org/compendia/cpc/ Commonwealth Agricultural Bureau International (CABI), Wallingford, UK. Date accessed 07 May 2010
Hooker WJ. (ed.). 1981. Compendium of Potato Diseases. American Phytopathological Society. St. Paul, Minnesota, USA. 125 pp.
Jeffries C. 1998. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Wale S, Platt HW (Bud), Cattlin N. 2008. Diseases, pests and disorders of Potatoes. A color Handbook. Manson Publishing, London, UK.176 pp.
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the safe movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Scientific name
Potato Leaf Roll Virus (PLRV)
Significance
PLRV is not considered a pathogen of quarantine status because of its wide distribution.
PLRV causes severe yield loss (up to 90%) and, in some cultivars quality reduction due to internal damage to tubers (net necrosis) (Jeffries, 1998). One estimate has suggested the virus is responsible for yield losses of 20 million tons globally (Wale, 2008).
Symptoms
Foliage: Primary symptoms develop in plants infected with the virus during the current season and are usually are restricted to pal discoloration or anthocianescence of the tops of the plants and slight leafrolling of the leaflets (Jeffries, 1998, Hooker, 1991). Some purple discoloration of affected leaflets may occur. (Wale, 2008) Symptoms may be absent, particularly if infection occurs late in the season.
Secondary symptoms develop in plants arising from infected tubers. Inward rolling of lower leaflets, extending ultimately to upper leaves, is typical. Rolled leaves are leathery and break easily when crushed between the fingers due to the excessive accumulation of carbohydrates. PLRV infected plants are usually stunted and erect and produce normal-shaped, but small, tubers (Wale, 2008). In some cultivars, severe chlorosis may develop in apical leaves. Conspicuous variations in symptoms can be observed in some genotypes (Jeffries, 1998).
Tubers: Some varieties may develop internal necrosis (net necrosis) in the tubers as a result of primary, secondary or tertiary infection with PLRV (Douglas and Pavek, 1972). Net necrosis may not be apparent at harvest but can develop in store (Wale, 2008).
Hosts
Solanum tuberosum , Ullucus tuberosus (Lizarraga, 1996), Capsella bursa-pastoris (Fox, 1993), Capsicum annuum and Lycopersicon esculentum has been reported as natural hosts.
About 20 species largely in the Solanaceae and a few non-solanaceous host including C. bursa-pastoris, Gomphrena and Montia perfoliata have been artificially infected (Jeffries, 1998).
Geographic distribution
Asia, Europe, North America, Central America, South America, Oceania
Biology and transmission
PLRV is transmitted by several aphid species in a persistent (circulative manner) principally by Myzus persicae. The virus can be spread long-distances by winged aphids, however, virus distribution occurs mainly through infected potato seed tubers and other vegetative parts including seedlings and micropropagated plants. PLRV has not been reported in true seed (CABI, 2007).
Detection/indexing method in place at CIP
- At CIP, the virus is detected by DAS-ELISA.
Treatment/control
- In seed certification schemes, no virus infections must be tolerated during the growing season. Stocks of in vitro cultures used for propagation should be from pathogen-free plants and maintained under conditions designed to prevent infection and contamination.
Procedure followed at CIP in case of positive test
- In CIP if pathogen is detected the imported germplasm must be cleaned by thermotherapy.
References of protocols at EPPO, NAPPO or other similar organization
NAPPO. 2003. Regional standard for Phytosanitary Measures (RSPM) No.3. Requirements for importation of potatoes into a NAPPO member country. 53 pp.
OEPP/EPPO. 1998. EPPO certification scheme for seed potatoes. Bulletin OEPP/EPPO Bulletin 28:561–567.
References and further reading
CABI. 2007. Crop Protection Compendium [online] Available from URL: www.cabi.org/compendia/cpc/ Commonwealth Agricultural Bureau International (CABI), Wallingford, UK. Date accessed 07 May 2010
Douglas DR, Pavek JJ. 1972. Net necrosis of potato tubers associated with primary, secondary and tertiary infection of leafroll. American Potato Journal, 49:330–333.
Fox L, Biever KD, Toba HH, Duffus JE, Thomas PE. 1993. Overwintering and monitoring of potato leafroll virus in some wild crucifers. American Potato Journal, 70(7):505-515.
Hooker WJ. (ed.). 1981. Compendium of potato diseases. American Phytopathological Society. St. Paul, Minnesota, USA. 125 pp.
Jeffries C. 1998. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Lizárraga C, Santa Cruz M, Salazar LF.1996. First report of potato leafroll luteovirus in ulluco (Ullucus tuberosus Caldas). Plant Disease, 80(3):344.
Wale S, Platt, HW (Bud), Cattlin N. 2008. Diseases, pests and disorders of Potatoes. A Color Handbook. Manson Publishing, London, UK.176 pp.
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Viroids - potato
Contributors to this section: CIP, Lima, Peru (Carols Chuquillanqui, Segundo Fuentes, Ivan Manrique, Giovanna Muller, Willmer Pérez, Reinhard Simon, David Tay, Liliam Gutarra); CIP, Nairobi, Kenya (Ian Barker); FERA, UK (Derek Tomlinson, Julian Smith, David Galsworthy, James Woodhall).
Spindle tuber of potato
Scientific name
Potato Spindle Tuber Viroid (PSTVd)
Significance
EPPO A2 quarantine organism.
Symptoms
Foliage: Symptom expression is influenced by the host species, host cultivar, strain of PSTVd, environmental conditions and method of inoculation (Harris, 1980; Pfannenstiel, 1980).
Stem and blossom pedicels are slender, longer than normal and remain erect. Leaflets are slightly reduced with fluted margins, tend to curve inward and overlap the terminal leaflet. Angles between stems and petioles are more acute than normal (Hooker, 1981; Wale, 2008). Infected plants are stunted and their leaves are dark green and rugose.
Tubers: Tubers may be elongated, with pointed ends and reduced in size (Jeffries, 1998). Tuber eyes appear to be more numerous and have characteristics of indentation or enhanced edges (Wale, 2008)
Hosts
Lycopersicon esculentum (tomato), Persea americana (avocado), Solanum tuberosum (potato), Ipomoea batatas (sweet potato), Solanum melongena (aubergine or eggplant), Solanum (nightshade), Solanum nigrum (black nightshade).
Geographic distribution
Asia, Europe, North America, Central America, South America, Oceania.
Biology and transmission
PSTVd is readily transmitted through botanical seed, pollen or ovules (Grassmick, 1986) and contact, but mainly by machinery in the field (Wale, 2008).
PSTVd is easily mechanically transmitted. It was found that transmission could occur when infectious sap contaminated cutting knives used for tuber propagation, with contamination of young tuber sprouts with infectious sap resulting in the highest percentage of infection. Heterologous encapsidation of PSTVd in particles of PLRV has been reported and may explain the observed insect transmission. This has important implications for epidemiology and spread of PSTVd in potato fields (Querci et al., 1997).
Detection/indexing method in place at CIP
- At CIP, the viroid is detected by NASH.
Treatment/control
- In seed certification schemes, no virus infections must be tolerated during the growing season. Stocks of in vitro cultures used for propagation should be from pathogen-free plants and maintained under conditions designed to prevent infection and contamination.
Procedure followed at the centers in case of positive test
- In CIP if pathogen is detected the imported germplasm must be cleaned by thermotherapy.
References of protocols at EPPO, NAPPO or other similar organization
OEPP/EPPO. 1978. Data sheets on quarantine organisms No. 97; Potato spindle tuber viroid. Bulletin OEPP/EPPO Bulletin, 8:2.
OEPP/EPPO. 2004. Potato spindle tuber pospiviroid. Diagnostic protocols for regulated pests. PM7/33(1). Bulletin OEPP/EPPO Bulletin 34:257-269.
References and further reading
Grasmick ME, Slack SA. 1986. Effect of potato tuber spindle viroid on sexual reproduction and viroid transmission in true potato seed. Canadian Journal of Botany 64:336–340.
Harris PS, Browning IA. 1980. The effects of temperature and light on the symptom expression and viroid concentration in tomato of a severe strain of potato spindle tuber viroid. Potato Research, 23(1):85–93
Hooker WJ. (ed.). 1981. Compendium of Potato Diseases. American Phytopathological Society. St. Paul, Minnesota, USA. 125 pp.
Pfannenstiel MA, Slack SA, 1980. Response of potato cultivars to infection by the potato spindle tuber viroid. Phytopathology, 70(9):922–926
Querci M, Owens RA, Bartolini I, Lazarte V, Salazar LF, 1997. Evidence for heterogeneous encapsidation of potato spindle tuber viroid in particles of potato leafroll virus. Journal of General Virology, 78(6):1207–1211.
Wale S, Platt HW (Bud), Cattlin N. 2008. Diseases, Pests and Disorders of Potatoes. A Color Handbook. Manson Publishing, London, UK. 176 pp.
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Bacteria - potato
Contributors to this section: CIP, Lima, Peru (Carols Chuquillanqui, Segundo Fuentes, Ivan Manrique, Giovanna Muller, Willmer Pérez, Reinhard Simon, David Tay, Liliam Gutarra); CIP, Nairobi, Kenya (Ian Barker); FERA, UK (Derek Tomlinson, Julian Smith, David Galsworthy, James Woodhall).
|
Contents: |
Bacterial wilt of potato; Potato brown rot
Scientific name
Ralstonia solanacearum (Smith 1896) Yabuuchi et al. 1996
Significance
EPPO A2 quarantine organism.
Race 3 (biovar 2A) strains of R. solanacearum, which affect mainly potato but occasionally tomato and other solanaceous crops and weeds, are most common in higher elevations of the tropics (up to 3400 masl). At lower elevations, race 1 strains are most prevalent and affect a wide range of crops and weeds. Crops highly susceptible to race 1 (biovars 1, 3 or 4) of R. solanacearum are potato, tobacco, tomato, eggplant, chili, bell pepper, and groundnut (peanut).
Symptoms
Foliage: Wilting (similar to lack of water), stunting and yellowing of the foliage. The browning of vascular bundles may be seen when the cortex is peeled. Characteristic, too, is the initial wilting of only part of the stems of a plant, or even one side of a leaf or stem. If disease development is rapid, the entire plant wilts quickly, without yellowing. Alternatively, the diseased stem can wilt completely and dry up, while the remainder of the plant appears healthy.
Tubers: External symptoms on the tuber are visible at harvest when infection is severe. Bacterial ooze often emerges from the eyes and stem-end attachment of infected tubers. Cut tubers show a pus-like slime coming out of the vascular ring with a slight squeezing or it may exude naturally. In advanced stages of the infection, tubers exhibit brownish discoloration of the vascular ring.
Hosts
Lycopersicon esculentum (tomato), Nicotiana tabacum (tobacco), Solanum melongena (aubergine, eggplant), Solanum tuberosum (potato), Musa spp. (banana), Musa paradisiaca (plantain), Heliconia, Solanum dulcamara, Anthurium spp., Arachis hypogea (groundnut, peanut), Capsicum annuum (bell pepper), Gossypium spp. (cotton), Hevea brasiliensis (rubber), Ipomoea batatas (sweet potato), Manihot esculenta (cassava), Ricinus communis (castor bean), Zingiber officinale (ginger), Solanum cinereum, Solanum nigrum , Galinsoga parviflora, G. ciliata, Polygonum capitata, Portulaca oleracea, Urtica dioica and S. nigrum.
Geographic distribution
Asia, Europe, Africa, North America, Central America, South America, Oceania.
Biology and transmission
Infected seed tubers are the main means of dissemination of R. solanacearum (particularly for race 3 strains). In cool conditions, such as tropical elevations above 2500 m, infected but symptomless plants may harbor the bacterium and transmit it to progeny tubers as latent infection, leading to severe disease outbreaks when grown at warmer locations (French, 1968; Kelman et al., 1994). The pathogen can survive in soil (mostly on plant debris) and in the rooting system and rhizosphere of many hosts (weeds, other host crops, potato volunteers (Moffett et al., 1981; Persley, 1986; Singh, 1995).
Race 3 may be spread in water when infected, S. dulcamara grows in water. The bacterium may subsequently be spread to other hosts when contaminated water is used for irrigation (Elphistone et al., 1998; Wenneker et al., 1999.).
Growing medium accompanying plants, seedlings and micropropagated plants are liable to carry propagules in trade and transport (CABI, 2007).
Detection/indexing method in place at CIP
- In potato tubers: NCM-ELISA (Enzyme Linked Immunoabsorbent Assay on Nitrocellulose Membrane)
-
In potato stems: DAS-ELISA (Double antibody sandwich enzyme-linked inmunoasorbent assay)
Real time PCR for confirming positive results obtained in NCM or DAS ELISA.
Modified Kelman’s medium with Tetrazolium chloride for detection and isolation.
Treatment/control
- In seed certification schemes, no bacterial wilt must be tolerated during the growing season. Stocks of in vitro cultures used for propagation should be from pathogen-free plants and maintained under conditions designed to prevent infection and contamination.
Procedure followed at CIP in case of positive test
- If pathogen is detected and cannot be eradicated, the germplasm must be destroyed. If the germplasm is scarce or unique, maintain it separately under containment so as not to present a risk to other germplasm.
References of protocols at EPPO, NAPPO or other similar organization
NAPPO. 2003. Regional standard for Phytosanitary Measures (RSPM) No.3. Requirements for importation of potatoes into a NAPPO member country. 53 pp.
OEPP/EPPO. 1978. Data sheets on quarantine organisms No. 58. Pseudomonas solanacearum. Bulletin OEPP/EPPO Bulletin 8(2).
OEPP/EPPO. 1990. Quarantine procedures No. 26. Pseudomonas solanacearum, inspection and test methods. Bulletin OEPP/EPPO Bulletin 20:255-262.
OEPP/EPPO. 2004. Ralstonia solanacearum. Bulletin OEPP/EPPO Bulletin 34: 327-329
USDA/APHIS/PPQ.2003. Pest Data Sheet. Ralstonia solanacearum race 3 biovar 2.
References and further reading
CABI. 2007. Crop Protection Compendium [online] Available from URL: www.cabi.org/compendia/cpc/ Commonwealth Agricultural Bureau International (CABI), Wallingford, UK. Date accessed 06 May 2010
Elphinstone JG, Stanford HM, Stead DE. 1998. Detection of Ralstonia solanacearum in potato tubers, Solanum dulcamara, and associated irrigation water. In: Prior P, Allen C, Elphinstone J. (eds.). Bacterial Wilt Disease: Molecular and Ecological Aspects. Berlin, Germany: Springer publishing, 133–139.
French ER, Sequeira L. 1968. Bacterial wilt or moko of plantain in Peru. Fitopatologia, 3:27–38.
Kelman A, Hartman GL, Hayward AC. 1994. Introduction. In: Hayward AC, Hartman GL. (eds.). Bacterial wilt: the Disease and its Causative Agent, Pseudomonas solanacearum. Wallingford, UK: CAB International, 1–7.
Moffett ML, Wood BA, Hayward AC. 1981. Seed and soil: sources of inoculum for the colonization of the foliage of solanaceous hosts by Pseudomonas solanacearum. Annals of Applied Biology, 98(3):403–411
Persley GJ. 1986. Ecology of Pseudomonas solanacearum, the causal agent of bacterial wilt. In: Persley GJ, ed. Proceedings of an International Workshop held at PCARRD, Los Baños, Philippines 8–10 October 1985. ACIAR Proceedings, 13, 71–76.
Samson R, Legendre JB, Christen R, Fischer-Le Saux M, Achouak W, Gardan L. 2005. Transfer of Pectobacterium chrysanthemi(Burkholder et al. 1953) Brenner et al. 1973 and Brenneria paradisiaca to the genus Dickeya gen, nov, as Dickeya chrysanthemicomb. nov. and delineation of four novel species, Dickeya dadantii sp. nov., Dickeya dianthicola sp. nov., Dickeya dieffenbachiaesp. nov. and Dickeya zeae sp. nov. International Journal of Systematic and Evolutionary Microbiology 55:1415–1427
Singh R. 1995. Seed transmission studies with Pseudomonas solanacearum in tomato and eggplant. ICIAR Bacterial Wilt Newsletter, 11:12-13.
Wenneker M, Verdel MSW, Groeneveld RMW, Kempenaar C, Beuningen AR, van Janse JD. 1999. Ralstonia (Pseudomonas) solanacearum race 3 (biovar 2) in surface water and natural weed hosts: First report on stinging nettle (Urtica dioica). European Journal of Plant Pathology, 105(3):307–315.
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the safe movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Specific references on detection:
NCN-ELISA kit for the detection of Ralstonia solanacearum in potato tubers
Priou S, Gutarra L, Aley P. 1999. Highly sensitive detection of Ralstonia solanacearum in latently infected potato tubers by post-enrichment ELISA on nitrocellulose membrane. EPPO Bulletin / Bulletin OEPP 29: 117–125.
Priou S, Salas C, de Mendiburu F, Aley P, Gutarra L. 2001. Assessment of Latent Infection Frequency in Progeny Tubers of Advanced Potato Clones Resistant to Bacterial Wilt: A New Selection Criterion. Potato Research 44: 359–373.
Priou S, Torres R, Villar A, Gutarra L, de Mendiburu F. 2001. Optimization of sample size for the detection of latent infection by Ralstonia solanacearum in potato seed tubers in the highlands of Peru. Potato Research 44: 349–358.
DAS-ELISA kit for the detection of Ralstonia solanacearum in potato stems before harvest of the potato crop
Priou S, Gutarra L, Aley P, de Mendiburu F, Llique R. 2009. Detection of Ralstonia solanacearum (Biovar 2A) in stems of symptomless plants before harvest of the potato crop using post-enrichment DAS-ELISA. Plant Pathology , in press
CIP's detection procedures for Ralstonia solanacearum is available online from URL:
http://www.cipotato.org/potato/pests_diseases/bacterial_wilt/detection_kits.asp Date accessed 06 May 2010
Real-time PCR for detection of Ralstonia solanacearum
Weller SA, Elphinstone JG, Smith NC, Boonham N, Stead DE. 2000a. Detection of Ralstonia solanacearum strains a quantitative, multiplex, real time, fluorogenic PCR (TaqMan) assay. Appl. Environ. Microbiol. 66:2853–2858.
Weller SA, Elphinstone JG, Smith NC, Stead DE. 2000b. Detection of Ralstonia solanacearum from potato tissue by post enrichment TaqMan PCR. EPPO Bull. 30:381–383.
Modified Kelman’s medium with Tetrazolium chloride for detection and isolation of Rs
French ER, Gutarra L, Aley P, Elphinstone J. 1995. Culture media for Pseudomonas solanacearum: isolation, identification and maintenance. Fitopatologia 30, 126–30.
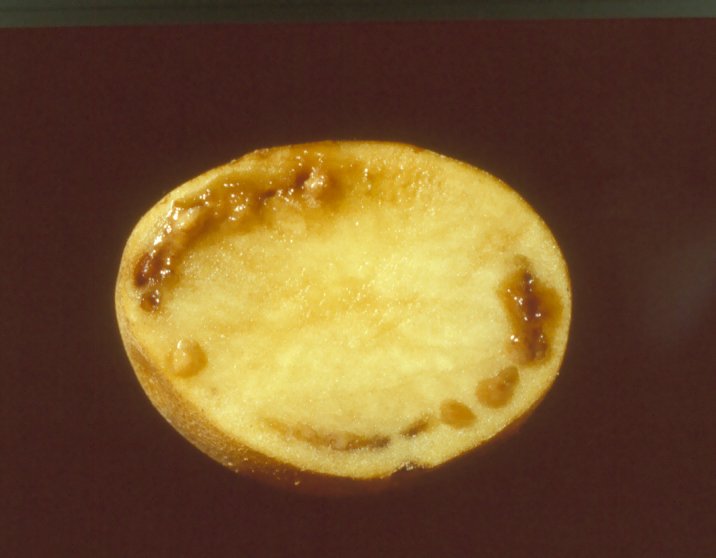 BW on tubers (photo: CIP) |
 Plants with BW symptom (photo: CIP) |
Scientific name
Dickeya chrysanthemi (Burkholder et al. 1953) Samson et al. 2005, comb. nov.
Significance
EPPO A2 quarantine organism.
Erwinia chrysanthemi has been reclassified into six new Dickeya species (Samson et al., 2005). The revised nomenclature of these pathogens has distinguished them from other soft rot erwiniae (including P. atrosepticum and P. carotovorum). Different biovars of D. chrysanthemi have been characterized by biochemical, physiological, serological, and molecular and pathogenicity tests. In potato, different Dickeya species have been found in Europe, viz. D. diathicola, D. dadantii and D. zeae (Wolf, 2007) and in other countries including Australia and Peru (Elphinstone, 2007). Furthermore, Dickeya strains have been found in potatoes grown in Israel, which could not be classified in any of the six new species (van der Wolf, 2007).
Symptoms
Foliage: Wilting and desiccation of foliage. In warmer parts causes increasing incidence of blackleg especially when temperature rises above 25°C, whereas P. atrosepticum typically causes blackleg symptoms under cool wet conditions. The foliar symptoms most commonly associated with D. dianthicola in warm dry growing conditions include brown staining of the vascular tissues and occasionally necrosis and hollowing of the stem, which usually remains green until leaf desiccation is complete (Elphinstone, 2007). Symptoms caused by D. dianthicola under warm dry conditions can be confused with those of the other wilting diseases.
Tubers: Symptoms of soft rot disease on potato tubers are similar whether caused by Dickyea or Pectobacterium spp. In cases of severe infection, progeny tubers are rotten in the soil.
Hosts
The pathogen has been reported worldwide on many hosts as Erwinia chrysanthemi.
Geographic distribution
Asia, Europe, Africa, North America, Central America, South America, Oceania
Biology and transmission
Seed tubers and seed pieces are the primary source of inoculum for Dickeya spp. Factors influencing disease development on potato caused by Dickeya spp. are generally the same as for P. atrosepticum, with the exception of temperature, where a warmer spring and summer favors disease development by Dickeya spp. (Elphinstone, 2007). D. dianthicola biovars 3 and 7 have been reported as more adapted to temperate climates while biovar 3 variant is more adapted to a higher temperature (van der Wolf, 2009; Tsror, 2009; Stead, 1999). Dickeya spp. is more efficient to colonize plant tissue compared to Pectobacterium spp., however it seems to act like biotrophic organism, which needs the host for long-term survival. Dickeya spp. survives poorly in free soil but has been frequently found in surface water, suggesting that it can persist for long periods in water (van der Wolf, 2009; Elphinstone, 2007). The bacteria can invade stems from the mother seed tuber, which can develop blackleg symptoms. The development of black leg depends on time at which the mother tuber rots. Invasions of tubers by bacteria can occur in a number of ways. Immature skins on tubers, wounding and high nitrogen fertilization predispose tubers to soft rot (Wale, 2008).
The bacterium is transmitted in soil and growing medium, and can survive for up to 10 weeks in cattle manure (Lohuis, 1990). Over long distances, and especially across national borders, it is mainly spread by infected vegetative propagating material (CABI, 2007).
Detection/indexing method in place at CIP
- Crystal violet pectato (CVP) medium for detection and isolation of D. chrysanthemi (Pérombelon and Burnet (1991).
- Modified Kelman’s without Tetrazolium chloride (CPG) medium for detection and isolation of D. chrysanthemi (French et al, 1995)
Treatment/control
- In seed certification schemes, no bacterial wilt must be tolerated during the growing season. Stocks of in vitro cultures used for propagation should be from pathogen-free plants and maintained under conditions designed to prevent infection and contamination.
Procedure followed at CIP in case of positive test
- If pathogen is detected and cannot be eradicated, the germplasm must be destroyed. If the germplasm is scarce or unique, maintain it separately under containment so as not to present a risk to other germplasm.
References of protocols at EPPO, NAPPO or other similar organization
NAPPO. 2003. Regional standard for Phytosanitary Measures (RSPM) No.3. Requirements for importation of potatoes into a NAPPO member country. 53 pp.
OEPP/EPPO. 1982. Data sheets on quarantine organisms. No. 53. Erwinia chrysanthemi. Bulletin OEPP/EPPO Bulletin 12 (1).
OEPP/EPPO. 1988. A1 and A2 lists of quarantine pests. Specific quarantine requirements. EPPO Publications Series B No. 92.
OEPP/EPPO. 1990. Specific quarantine requirements. EPPO Technical Documents No. 1008.
References and further reading
CABI. 2007. Crop Protection Compendium [online] Available from URL: www.cabi.org/compendia/cpc/ Commonwealth Agricultural Bureau International (CABI), Wallingford, UK. Date accessed 06 May 2010
Elphinstone J. 2007. Growers’ advice: Erwinia chrysanthemi (Dickeya spp.): What it is, and what you can do. British Potato Council.
Lohuis H. 1990. Does liquid manure spread weeds and bacteria? PSP Pflanzenschutz Praxis, No. 3:28–30.
Samson R, Legendre JB, Christen R, Fischer-Le Saux M, Achouak W, Gardan L. 2005. Transfer of Pectobacterium chrysanthemi (Burkholder et al. 1953) Brenner et al. 1973 and Brenneria paradisiaca to the genus Dickeya gen, nov, as Dickeya chrysanthemi comb. nov. and delineation of four novel species, Dickeya dadantii sp. nov., Dickeya dianthicola sp. nov., Dickeya dieffenbachiae sp. nov. and Dickeya zeae sp. nov. International Journal of Systematic and Evolutionary Microbiology 55:1415–1427
Stead D. 1999. Bacterial diseases of potato: relevance to in vitro potato seed production. Potato Research 42: 449–456
Tsror (Lahkim) L, Erlich O, Lebiush S, Hazanovsky M, Zig U, Slawiak M, Grabe G, van der Wolf JM, van de Haar JJ. 2009. Assessment of recent outbreaks of Dickeya sp. (syn. Erwinia chrysanthemi) slow wilt in potato crops in Israel. European Journal of Plant Pathology 123 (3):311–320
Wale S, Platt HW (Bud), Cattlin N. 2008. Diseases, Pests and Disorders of Potatoes. A Color Handbook. Manson Publishing, London, UK. 176 pp.
van der Wolf JM, Czajkowski R, Velvis H. 2009. Why is Dickeya spp. (syn. Erwinia chrysanthemi) taking over? – The ecology of a blackleg pathogen. In: Symposium KNPV Pests and climate change, 3 December, 2008, Wageningen, The Netherlands
van der Wolf JM, Speksnijder A, Velvis H, van der Harr J, van Doorn J. 2007. Why is Erwinia chrysanthemi (Dickeya sp.) taking over? – The ecology of a blackleg pathogen. In: Hannukkala, A. and M. Segerstedt (eds.). 2007. New and old pathogens of potato in changing climate. Proceedings of the EAPR pathology Section Seminar. 2–6 Th. July 2007, Hattula, Finland.
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the safe movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Specific references on detection:
Pérombelon MCM, Burnett EM. 1991. Two modified crystal violet pectate (CVP) media for detection, isolation and enumeration of soft rot erwinias. Potato Research 34: 79-85.
Modified Kelman’s without Tetrazolium chloride (CPG) medium for detection and isolation of D. chrysanthemi
French ER, Gutarra L, Aley P, Elphinstone J. 1995. Culture media for Pseudomonas solanacearum: isolation, identification and maintenance. Fitopatologia 30, 126-30.
Scientific name
Clavibacter michiganensis subsp. sepedonicus (Spieckermann & Kotthoff) Dye & Kemp
Significance
EPPO A2 quarantine organism.
Symptoms
Foliage: The symptoms develop from mid to late season and usually appear on the lower leaves which are slightly rolled at the margins and are pale green color. As wilting progresses, bars of bright yellow tissue develop between veins. Symptoms may occur on only one or a few stems of a plant and proceed upwards from the lower leaves until the entire stem is wilted. Severely infected plants die prematurely. Potato cultivars vary greatly in their propensity to show symptoms. Symptomless foliage may harbor latent infections especially under cooler conditions. A milky white exudate can be squeezed of stems when cross sectioned at their base.
Tubers: Symptoms are visible at harvest or storage, these begin at stolon end. Discoloration of the vascular tissue is characteristic of this disease; it varies from creamy-yellow to brown areas. When the cut tuber is pressured, creamy odorless ooze may be expressed from the tissue. As the disease progresses, the tissue rounding the vascular ring becomes corky-brown and may develops cavities. Secondary infections increases rotting and tuber disintegrates. Externally, the tubers form diseased plants may appear normal. Sometimes reddish to brown blotches and/or surface cracks are present especially near the eyes. Tuber symptoms may be confused with those caused by the bacterium Ralstonia solanacearum.
Symptomless tubers may harbor latent infections.
Hosts
Lycopersicon esculentum (tomato), Lycopersicon pimpinellifolium (currant tomato), Solanum melongena (aubergine, eggplant), Solanum tuberosum (potato), sugarbeet seed and roots
Geographic distribution
Asia, Europe, Africa, North America, Central America, South America
Biology and transmission
The pathogen overwinters mostly in infected tubers and persists on equipment, machinery and storage (crates, sacks, bags, bins, barrels, etc.) as dried slime (Hooker, 1981, Van der Wolf, 2005). It can overwinter in volunteer plants, in cull piles or in debris from infected crops (Wale et al, 2008). Infection occurs through tuber wounds and invades the xylem vessels and later xylem parenchyma and adjacent tissue and cause separation at the vascular ring (Hooker, 1981). The bacteria migrate from the seed tuber to the stems via the vascular tissue, and subsequently into progeny tubers through the stolons. Disease spread is most frequent when seed tubers are cut and bacteria from infected tubers are transferred onto freshly cut seed surfaces (Wale et al., 2008). Latent pathogen populations in symptomless in vitro plantlets of some potato cultivars can survive several generations (Jeffries, 1998). C. michiganensis subsp. spedenicus is liable to carry in growing medium accompanying plants (CABI, 2007).
Detection/indexing method in place at CIP
- Real time PCR: NCP-88 medium without antibiotics for isolation
Treatment/control
- If pathogen is detected the imported germplasm must be destroyed according Peruvian laws. C. michiganensis subsp. sepedonicus is catalogued as A1 quarantine pathogen for Peru.
Procedure followed at CIP in case of positive test
- If pathogen is detected and cannot be eradicated, the germplasm must be destroyed. If the germplasm is scarce or unique, maintain it separately under containment so as not to present a risk to other germplasm.
References of protocols at EPPO, NAPPO or other similar organization
NAPPO. 2003. Regional standard for Phytosanitary Measures (RSPM) No.3. Requirements for importation of potatoes into a NAPPO member country. 53 pp.
OEPP/EPPO. 2004. Clavibacter michiganensis subsp. sepedonicus. Bulletin OEPP/EPPO Bulletin 34: 323–325
OEPP/EPPO. 2006. Clavibacter michiganensis subsp. sepedonicus. Bulletin OEPP/EPPO Bulletin 36: 99–109
References and further reading
CABI. 2007. Crop Protection Compendium [online] Available from URL: www.cabi.org/compendia/cpc/ Commonwealth Agricultural Bureau International (CABI), Wallingford, UK. Date accessed 06 May 2010
EPPO/CABI. 1992. Pseudomonas solanacearum. In: Smith IM, McNamara DG, Scott PR, Harris KM (E KM, eds.) Quarantine Pests for Europe. Wallingford, UK: CAB International.
Franc GD. 1999. Persistence and latency of Clavibacter michiganensis subsp. sepedonicus in field-grown seed potatoes. Plant Disease, 83(3):247-250.
Hooker WJ. (ed.). 1981. Compendium of Potato Diseases. American Phytopathological Society. St. Paul, Minnesota, USA. 125 pp.
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the Safe Movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
van der Wolf JM, Elphinstone JG, Stead DE, Metzler M, Müller P, Hukkanen A, Karjalainen R. 2005. Epidemiology of Clavibacter michiganensis subsp. sepedonicus in relation to control of bacterial ring rot. Report 95. Plant Research International. Wageningen, The Netherlands. 38 pp.
Wale S, Platt HW (Bud), Cattlin N. 2008. Diseases, Pests and Disorders of Potatoes. A Color Handbook. Manson Publishing, London, UK. 176 pp.
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Specific references on detection:
Real-time PCR for detection of Clavibacter michiganensis subsp. Sepedonicus
Shaad W, Berthier-Shaad Y, Sechler A, Knorr D. 1999. Detection of Clavibacter michiganensis subsp. Sepedonicus in potato tubers by Bio PCR and automated real-time fluorescence detection system. Plant Disease 83, 1095–1100.
NCP-88 medium without antibiotics for isolation
OEPP/EPPO. 2006. Clavibacter michiganensis subsp. sepedonicus. Bulletin OEPP/EPPO Bulletin 36: 99–109
Phytoplasma - potato
Contributors to this section: CIP, Lima, Peru (Carols Chuquillanqui, Segundo Fuentes, Ivan Manrique, Giovanna Muller, Willmer Pérez, Reinhard Simon, David Tay, Liliam Gutarra); CIP, Nairobi, Kenya (Ian Barker); FERA, UK (Derek Tomlinson, Julian Smith, David Galsworthy, James Woodhall).
Potato purple-top wilt phytoplasma
Scientific name
Potato purple-top wilt
Six phytoplasmas on potato has been distinguished:
Potato witches' broom phytoplasma
Potato marginal flavescence phytoplasma
Potato purple toproll phytoplasma
Potato phyllody phytoplasma
Potato stolbur phytoplasma
Potato purple-top wilt phytoplasma
Significance
Potato purple-top wilt phytoplasma is EPPO A1 quarantine organism.
Hosts
Potato purple-top wilt phytoplasma is closely related to the aster yellows phytoplasma complex which has a very wide host range. About 350 species from at least 54 plant families are susceptible. Potatoes are not favored hosts (Wright et al., 1983). It is difficult to state categorically what is the host range of the phytoplasmas of this group found in potato.
Geographic distribution
North America (Lee et al., 2006)
Europe (Russia)(Girsova et al., 2008)
Biology and transmission
The principal vector is the leafhopper Macrosteles fascifrons, in which the phytoplasmas are propagative. The vectors remain infective for life. They can
feed on a variety of plants, and the apparent host range of the phytoplasma depends more on the host preferences of the vector than on any particular specificity between plant and phytoplasma.
Detection/indexing method in place at CIP
- At CIP, detection methodology is being improved
Treatment/control
- In seed certification schemes, no virus infections must be tolerated during the growing season. Stocks of in vitro cultures used for propagation should be from pathogen-free plants and maintained under conditions designed to prevent infection and contamination.
Procedure followed at the centers in case of positive test
- In CIP if pathogen is detected the imported germplasm must be cleaned by thermotherapy.
References of protocols at EPPO, NAPPO or other similar organization
CABI/EPPO. Data sheets on Quarantine pest: Potato purple-top wilt phytoplasma. Prepared by CABI and EPPO for the European Communities. 5 p.
References and further reading
Girsova N, Bottner KD, Mozhaeva KA, Kastalyeva TB, Owens RA, Lee I. 2008. Identification of Phytoplasma Species Associated With Potato Diseases in Russia. International Symposium on Crop Protection. 73 (2): 331–333.
Lee IM, Bottner KD, Secor G, Rivera-Varas V. 2006. "Candidatus Phytoplasma americanum", a phytoplasma associated with a potato purple top wilt disease complex. Int. J. Syst. Evol. Microbiol. 56 (7):1593–1597.
Wright NS, Raine J, Valenta V. 1983. Mycoplasmas. In: Compendium of potato diseases (Ed. by Hooker, W.J.), pp. 91–93. American Phytopathological Society, St. Paul, Minnesota, USA.
Younkin SG. 1943. Purple-top wilt of potatoes caused by the aster yellows virus. American Journal of Potato Research 20 (7):177–183.
Seed Health General Publication Published by the Center or CGIAR
Jeffries C. 1998. FAO/IPGRI Technical guidelines for the safe movement of Germplasm. No. 19. Potato. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
More Articles...
- Fungi - potato
- Diagnostic methods for determining the health of germplasm of clonal crops
- Guidelines for the safe transfer of potato germplasm
- Diagnostic protocols for sweetpotato
- Case studies - CIP potato and sweet potato
- CIP germplasm health
- Germplasm health testing validation data for potato
- Germplasm health testing validation data for sweet potato
- General principles underpinning safe transfer of tissue cultured clonal germplasm
- Pest list information



 stog-clonal
stog-clonal
