CGKB News and events stog-faba-bean
Viruses - faba bean
Contributors to this section: ICARDA, Syria (Siham Asaad, Abdulrahman Moukahal).
Scientific name
Bean Yellow Mosaic Virus(BYMV)
Other scientific names
Bean virus 2, Canna mosaic virus ,Gladiolus mosaic virus, Gloriosa stripe mosaic virus.
Significance
During pulse surveys from 2000-2005, BYMV infections were uncommon and found in less than 1% of surveyed crops. However, BYMV was sometimes found in faba bean crops and occasionally in peas with within crop virus incidences of 1-15% and up to 7% respectively. In NSW, a small survey showed that faba bean crops had an average within crop incidence of BYMV of 26% of plants (ranging from 1-63%) (van Leur et al. 2002). Field surveys in 1998-1999 showed that some faba bean and field pea crops were infected with BYMV and the within crop virus incidences were 1-31% and 1-11% respectively. Plot trials showed that lupins infected with the necrotic strain of BYMV can have grain yields reduced by 95%.
Symptoms
Leaves of infected plants show mild mosaic followed by narrowing. The new growth from leaf axils has leaves that are narrow, elongated and light green. Early infections adversely affect plant growth and yield.
Lentils develop mild mosaic, light green or yellow leaves, reduction in leaf size and stunting may occur. Infected plants produce very little seed.
Narrow-leaf lupins, infected with the necrotic strain of BYMV initially develop yellow leaves followed by necrosis of growth tips and plant death. Non-necrotic strains of BYMV cause yellowing and dwarfing but do not cause death of the plant.
Subterranean clover plants develop leaf mottling, leaf deformation and distinct yellowing between the veins. Plants become dwarfed and symptoms usually occur in patches and along the edges of paddocks.
Hosts
The host range of BYMV is wide and not limited to Fabaceae. The virus is reported to infect nearly 200 species in 14 families. Temperate pulse hosts include chickpeas, faba beans, field peas, lentils and lupins. Temperate legume pasture hosts include lathyrus, lucerne, vetch and medic and clover species. BYMV has a number of sub-tropical and tropical pulse hosts, including soybeans, peanuts and French beans as well as legume pasture hosts. It also infects ornamental hosts, the most common being gladiolus species.
Geographic distribution
Belarus, Belgium: Bulgaria, Czech Republic,Denmark, Finland, Former Yugoslavia, France, Germany, Greece, Hungary, Lithuania, Netherlands, Poland, Portugal, Romania, Russian Federation, Russia (Europe),Russian Far East, Spain, Sweden, Ukraine, United Kingdom, China, Georgia (Republic), India , Indonesia, Iran, Israel, Japan, Jordan, Kazakhstan, Korea, Lebanon, Pakistan, Syria, Turkey, Uzbekistan, Egypt, Kenya, Libya, Morocco, South Africa, Sudan, Tanzania, Tunisia Zambia, Zimbabwe, Western Hemisphere, Canada, Chile, Dominican Republic, Jamaica, Mexico, Montserrat, Peru, USA, Alabama, Kentucky, Tennessee, Australia, New Zealand.
Biology and transmission
BYMV is transmitted by more than 50 aphid species in a non-persistent manner: the main species are Acyrthosiphon pisum, Aphis fabae, A. gossypii, Aulacorthum solani Brevicoryne brassicae, Myzus persicae and Rhopalosiphum maidis. During surveys in Victoria the following BYMV vectors in pulse crops were found : Acyrthosiphon kondoi, Aphis craccivora, Aulacorthum solani, Brevicoryne brassicae and Myzus persicae. Acyrthosiphon kondoi, Aphis craccivora, Myzus persicae, Brachycaudus rumexicolens, Lipaphis erysimi, Rhopalosiphum maidis, R. padi, Sitodion miscanthi and Therioaphis trifolii have been reported as BYMV vectors.
The virus is also transmitted through seed of most temperate pulses, including faba beans, field peas, lentils, and lupins and through seed of a number of forage legumes and clovers. In Victoria, 18% of lentil seed lots tested had BYMV infections of 0.1-0.9%. In WA, the following BYMV seed transmission is reported: yellow and white lupins 3-6%, field peas 0.3-0.8%, faba beans 0.4%, lathyrus 0.1-0.2% and vetch 0.5%.
Seed transmission of BYMV in medics and clovers has also been reported in WA as follows: Melilotus indica (0.5%), Medicago polymorpha (0.9%), M. truncatula (0.3%), M. indica (1%), Trifolium arvense (0.1%), T. campestre (0.2%) and T. glomeratum (0.05%).
Detection/indexing method at ICARDA
- TBIA (Tissue Blot Immuno Assay test), Enzyme-linked immunosorbent assay (ELISA)
Treatment/control
- Seed is considered to be one of the main sources of BYMV, therefore sowing virus tested seed is recommended and commercial seed tests are available.
- BYMV causes heavy losses in narrow leafed lupins, so only virus tested seed is recommended for sowing. Virus resistant lupin varieties are now available.
- BYMV-infected pastures are another major source of the virus, which is then spread to crops by aphids. Chemical control of aphids is not an effective method for controlling non-persistently transmitted viruses such as BYMV.
- Pulse crops should be sown away from legume pastures to minimise the spread of BYMV.
- The spread of virus can also be reduced by controlling weed hosts from in and around paddocks.
Procedure followed at the centers in case of positive test
- Destroying seed, production healthy seed
References and further reading
Temperate Pulse Viruses: Bean Yellow Mosaic Virus (BYMV)
http://www.staff.kvl.dk/~thluj3/symptoms/fababymv.html
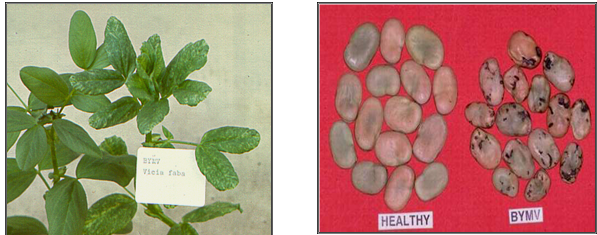 BYMV (photos:www.staff.kvl.dk and www.dpi.vic.gov.au) |
Scientific name
Broad Bean Stain Virus (BBSV).Lloyd, Smith & Jones, 1965.
Other scientific names
Broad bean F1 virus, Broad bean Evesham stain virus
Significance
Nineteen genotypes of lentil were studied in Syria for variability in seed transmission rates and yield losses induced by broad bean stain virus (BBSV) infection. Seed transmission rates of BBSV varied between 0.2% and 32.4%, whereas yield loss due to infection varied between 14% and 61%.
Symptoms
The disease is characterized by a mild mottling on the leaves.
The affected plants are reduced in growth, which is easily recognized, especially when they are compared with healthy plants. Yield reduction of 15-16% could occur in some different cultivars. Seed from infected plants occasionally show dark staining on the seed.
Hosts
Hosts restricted to Leguminosae; inoculated 44 species in 19 dicotyledonous families, but the virus infected only 7 species of legume: Crotalaria spectabilis, Lupinus hirsutus, Melilotus alba, Phaseolus vulgaris (French bean), Pisum sativum (pea), Trifolium incarnatum (crimson clover) and Vicia faba (broad bean).
Geographic distribution
Europe, Austria, Hungary, Italy, Poland, United Kingdom, Asia (as a whole), China, Syria, Turkey, Africa (as a whole) Egypt.
Biology and transmission
Transmission by Vectors: Transmitted by a vector; an insect; Apion vorax, Sitona spp.; Coleoptera.
Transmission through Seed: Common in certain varieties of broad bean, transmitted to up to 10% of the progeny of infected plants.
Transmission by Dodder: Not tested.
Virus transmitted by mechanical inoculation; transmitted by grafting; transmitted by seed (up to 10% in some Vicia faba cultivars).
Detection/indexing method at ICARDA
- Tissue Blot Immuno Assay test (TBIA), Enzyme-linked immunosorbent assay (ELISA).
Treatment/control
- For the three seed-borne viruses (BBSV, PSbMV and CMV) it is important to avoid the use of virus-infected seed lots.
- Rogue early infected plants to prevent further disease spread.
- For the aphid-transmitted viruses, spray systemic aphidicides, especially in lentil nurseries or seed multiplication plots. This should be considered in situations where winged forms of aphids begin to appear. Spraying will help suppress aphid populations in lentil fields.
- For BBSV, which is transmitted by weevils, treat seed with promet (12 ml/kg seed) to help reduce the vector and hence disease spread.
- All these viruses have a wide host range including other legumes such as dry peas, phaseolus bean, faba bean, alfalfa and clover.
Procedure in case of positive test
- Destroying infected seed, producing healthy seed
References and further reading
http://www.dpvweb.net/dpv/showdpv.php?dpvno=029
http://image.fs.uidaho.edu/vide/descr112.htm#Taxonomy
http://www.icarda.org/Publications/Field_Guides/Lentil/Lent7.Html
http://www.cababstractsplus.org/abstracts/Abstract.aspx?AcNo=19916777532
http://www.padil.gov.au/pbt/index.php?q=node/20&pbtID=152
 BBSV (photos:www.padil.gov.au) |
Scientific name
Pea Seed-borne Mosaic Virus (PSbMV)
Other scientific names
Pea fizzle top virus, Pea leaf rolling virus, Pea leaf roll mosaic virus, Pea leaf rolling mosaic virus.
Significance
PSBMV is of economic importance in pea, faba bean and lathyrus, mainly due to its effect on seed quality. It has been mistakenly considered to be a minor disease because it often causes only minor yield loss and mild symptoms, However, at the International Centre for Agriculture in the Dry Areas (ICARDA), Syria, glasshouse studies on yield losses due to PSBMV in chickpea, faba bean, lentil and pea showed losses of 66%, 40.5%, 44.6% and 49.2% respectively. Surveys of pulse crops over a number of years in Vic and SA showed that up to 15% of crops were infected with PSBMV, with a within crop incidence of up to 5% of plants. In surveys in WA in 1999, PSBMV was found in 42% of field pea crops and the level of virus detected in pea seed stocks was up to 63%. The surveys indicated that PSBMV has a severe effect on seed quality of pulse crops with seed coat symptoms evident on >80% of faba bean, >50% of lathyrus species and field peas and 5% of chickpea seed.
Symptoms
The disease is characterized by a mild mottling on the leaves, which is not easily recognizable because of the small size of the lentil leaf.
The affected plants are reduced in growth, which is easily recognized, especially when they are compared with healthy plants. Yield reductions of 15-16% could occur in some different cultivars. Seeds from infected plants occasionally show dark staining on the seed.
Lentils may show no symptoms or there may be chlorosis in new shoots, mottling on leaves, shoot tip necrosis and stunting of plants.
Hosts
The natural host range of PSBMV is limited to the Fabaceae. It infects temperate pulses (chickpea, faba bean, field pea, lentil) other legumes (garden pea, narbon bean) and pastures (lathyrus and vetch). A number of PSBMV pathotypes have been recognised by their ability to infect a number of pea differential genotypes.
Geographic distribution
Belgium, Bulgaria, Czechoslovakia (former -), Denmark, Finland, France, Germany, Netherlands, Poland, Romania, Russia (Europe), Sweden, Switzerland, United Kingdom, China, Taiwan, India, Israel, Japan, Jordan, Lebanon, Nepal, Syria, Turkey, Egypt, Ethiopia, Libya, Morocco, South Africa, Sudan, Tanzania, Tunisia, Zambia, Zimbabwe, Western Hemisphere, Brazil, Canada, Peru, USA, Washington Australia , New Zealand.
Biology and transmission
The virus is believed to have spread worldwide through the exchange of infected seed. Seed transmission rates of up to 100% in peas and up to 44% in lentils have been reported. In Victoria, we have detected PSBMV at low levels in some commercial chickpea seedlots (0.4% of seed) and at higher levels in field pea and lentil seedlots (greater than 2% of seed). In the USA, 3% PSBMV infection in pea seedlots and 32-40% in lentil seedlots have been reported. At ICARDA, PSBMV was found to be transmitted through lentil seeds at rates of up to 44%. PSBMV is also transmitted in a non-persistent manner by more than 20 aphid species and by mechanical means. The most efficient vector is the pea aphid (Acyrthosiphon pisum). The other species are as follows: A. pelargoni, A. sesbaniae, Aphis craccivora, A. fabae, A. gossypii, A. nasturtii, Aulacorthum circumflexum, A. solani, Brevicoryne brassicae, Cryptomyzus ribis, Dactynotus escalantii, Macrosiphum avenae, M. euphorbiae, M. pisi, M. rosae, Metopolophium dirhodum, Myzus persicae, Ovatus crataegarius, Phorodon cannabis, Rhopalosiphum padi and Semiaphis dauci. In Victorian pulse crop surveys, we have found cowpea aphid (Aphis craccivora), foxglove aphid (Aulacorthum solani), and green peach aphid (Myzus persicae), which are all vectors of PSBMV.
Detection/indexing method in place at the CGIAR Center at ICARDA
- (TBIA) Tissue Blot ImmunoAssay test, Enzyme-linked immunosorbent assay (ELISA)
Treatment/control
- Seed is considered to be the main source of PSBMV, therefore sowing virus tested seed is the most effective way of controlling this virus.
- Chemical control of aphids is not an effective method for controlling non-persistently transmitted viruses, such as PSBMV.
- Pulse crops should be sown away from other legumes to minimise the spread of PSBMV.
Procedure followed at the centers in case of positive test
- Destroy seed, produce healthy seed
Referencs and further reading
Temperate Pulse Viruses: Pea Seedborne Mosaic Virus (PSBMV)
http://www.agls.uidaho.edu/ebi/vdie//vide/descr575.htm
 PSbMV (photos:www.dpi.vic.gov.au) |
Scientific name
Broad Bean Mottle Virus (BBMV)
Significance
The virus was seen as being of minor importance until reports of its widespread occurrence began to appear in the 1970s and 1980s.
Symptoms
Plants display leaf symptoms, dwarfing and production of few or no flowers. Infected plants are in concentric patches. BBMV can affect seed quality by causing necrosis and shrivelling of the seed. Seed transmission rates in legumes are low and range from 0.1% to 1.4%.
 Broad Bean Mottle (photo: www.padil.gov.au) |
Hosts
Under experimental conditions susceptibility to infection by virus is found in several families. Susceptible host species are found in the Family Amaranthaceae, Chenopodiaceae, Leguminosae-Papilionoideae, Solanaceae. The following species were susceptible to experimental virus infection: Chenopodium album, Chenopodium amaranticolor, Chenopodium hybridum, Chenopodium quinoa, Cyamopsis tetragonoloba, Glycine max, Gomphrena globosa, Lathyrus odoratus, Lens culinaris, Lupinus albus, Medicago sativa, Melilotus albus, Nicotiana clevelandii, Phaseolus vulgaris, Pisum sativum, Trifolium hybridum, Trifolium incarnatum, Trifolium pratense, Trifolium repens, Trifolium subterraneum, Vicia faba, Vicia villosa.
Geographic distribution
BBMV was reported in faba bean crops in Portugal, Sudan, Morocco, and Algeria. then undertook a regional survey and found BBMV in faba bean crops in Egypt, Morocco, Sudan, Syria and Tunisia. Fortass and Bos (1991) surveyed faba bean crops in Morocco and found that luteoviruses and BBMV were the most prevalent viruses.
Biology and transmission
BBMV survives between growing seasons of the primary pulse host in an alternative host or in infected seed. Due to the fact that the natural host range includes at least four families and includes many food legumes and non-legume wild hosts, it may survive in a range of alternative hosts.
Virus is transmitted by a vector. Virus is transmitted by mechanical inoculation; transmitted by grafting.
Virus is transmitted by arthropods, by insects of the order Coleoptera; Acalymma trivittata, Colaspis flavida, Diabrotica undecimpunctata, Sitona lineata.
Detection/indexing method in place at the CGIAR Center at ICARDA
- Tissue Blot Immuno Assay test (TBIA), Enzyme-linked immunosorbent assay (ELISA)
Treatment/control
- Seed is considered to be the main source of BBMV, therefore sowing virus tested seed is the most effective way of controlling this virus.
- Commercial seed tests are available.
- Chemical control of insects is not an effective method for controlling virus.
Procedure in case of positive test
- Destroying seed, producing healthy seed under controlled conditions
References and further reading
http://www.padil.gov.au/pbt/index.php?q=node/46&pbtID=152
http://www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/00.010.0.03.002.htm
Scientific name
Pea Early-Browning Virus(PEBV)
Other scientific name
Broad Bean Yellow Band Virus (BBYBV)
Significance
Not Significant.
Symptoms
The symptoms are systemic necrotic spots, streaks and mosaic, leaves malformed, plants stunted.
Hosts
Families containing susceptible hosts: Alliaceae, Chenopodiaceae, Compositae, or Cruciferae, Gramineae, Leguminosae-Papilionoideae, Solanaceae. Species inoculated with virus that do not show signs of susceptibility: Allium cepa, Avena sativa, Beta vulgaris, Brassica campestris ssp. rapa, Hordeum vulgare, Lactuca sativa, Lycopersicon esculentum, Medicago sativa, Nicotiana glutinosa, Solanum tuberosum, Trifolium incarnatum, Trifolium pratense, Trifolium repens, Triticum aestivum, Vicia faba.
Geographic Distribution
The virus occurs in Belgium, Italy, Morocco, the Netherlands, Sweden, and the United Kingdom.
Biology and transmission
Virus is transmitted by a vector. Virus is transmitted by mechanical inoculation; transmitted by seeds (37% in Pisum sativum cv. Rondo).
Vector Transmission: Virus is transmitted by nematodes; family Trichodoridae; Trichodorus teres, T. pachydermus in The Netherlands; T. anenome, T. primitivus, T. viruliferus
Detection/indexing method
- Not important (TBIA) Tissue Blot ImmunoAssay test, Enzyme-linked immunosorbent assay (ELISA)
Treatment/control
Not given.
Procedure followed at the centers in case of positive test
Not given
References and further reading
http://www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/00.072.0.01.002.htm
Scientific name
Red Clover Vein Mosaic Virus (RCVMV)
Other scientific name
Pea Stunt Virus
Significance
It reduces the yield of red clover by reducing the foliage growth, decreasing persistence and increasing susceptibility to root rots.
Symptoms
RCVMV is reported to cause stunting in faba beans and inoculated plants have been reported to show chlorotic lesions or general chlorosis, tip mottle and abscission.
Hosts
It also infects a number of temperate pulses including Cicer arietinum (chickpea), Lathyrus odoratus (sweet pea), Lens culinaris (lentil), Pisum sativum (field pea), and Vicia faba (faba bean, broad bean, tick bean). RCVMV is seedborne in Trifolium pratense (red clover), Pisum sativum and Vicia faba.
Geographic distribution
It occurs in North America, South Africa, Europe, and India.
Biology and transmission
- Virus is transmitted by a vector. Virus is transmitted by mechanical inoculation; transmitted by seeds (in Trifolium pratense and Vicia faba, not transmitted by pollen.
- Vector Transmission: Virus is transmitted by arthropods, by insects of the order Hemiptera, family Aphididae; Acyrthosiphon pisum, Myzus persicae, Therioaphis (Pterocallidium) maculatus, Cavariella aegopodii, C. theobaldi. Virus is transmitted in a non-persistent manner.
Detection/indexing method in place at the CGIAR Center at ICARDA
- Not important, (TBIA) Tissue Blot Immuno Assay test, Enzyme-linked immunosorbent assay (ELISA)
Treatment/control
- Use resistant varieties.
- Resistance to vectors is another approach to controlling viruses.
References and further reading
http://www.padil.gov.au/pbt/index.php?q=node/46&pbtID=172
http://www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/00.056.0.04.027.htm
http://www.apsnet.org/online/feature/plantdisease/text/fig08.htm
http://www1.agric.gov.ab.ca/$Department/deptdocs.nsf/All/prm7877?OpenDocument
Insects - faba bean
Contributors to this section: ICARDA, Syria (Siham Asaad, Abdulrahman Moukahal)
|
Contents: |
Scientific names
Bruchus rufimanus Boheman, 1833 & Bruchidius incarnatus Bohemann, 1833.
Significance
Beans may contain un-emerged pupae or adults. High levels of damage or infestation are unacceptable for export outlets for human consumption. For seed purposes, the damage may not be as serious, although germination of the seed may be slightly reduced.
Symptoms (Damage)
Damage to beans is characterized by a circular hole in the seed where the adult beetles emerge. Other types of damage such as seed staining and smaller holes may be seen where the larvae has not matured.
Hosts
Faba bean, cowpea, common bean.
Geographic distribution
Widespread in all varieties of winter and spring field beans and also broad beans.
Biology and transmission
Bruchus spp. adults emerge from hibernation in spring and after feeding on pollen and nectar lay eggs on the immature faba bean pods. The larvae bore a small round hole through the pod coat and enter the first avail able developing seed. Most of the larval development and pupation occurs in the hard seeds after harvest in the store. The mature larvae bite a hole from inside almost to the seed surface leaving only a transparent epidermal seed membrane, which are called ‘windows’. The adults remain in the seeds and only emerge through the ‘windows’ after sowing. If the seeds are not sown, but stored for another season the adults will die.
Detection/indexing method in place at the CGIAR Center at ICARDA
- Visual inspection
Treatment/control
- Since the infestation of Bruchus spp. starts in the field control methods should aim at reducing the population in the field before the larvae penetrate the seed.
-
Several cultural measures can be used to reduce Bruchus spp. infestations:
- Deep ploughing after harvest.
- Use of uninfected or treated seeds for sowing which has to be followed by all farmers to avoid immigration of the flying beetles from infested to uninfected fields.
- Storage of seeds for 2 successive seasons, so that all adults are dead at the time of sowing.
- Late sowing also reduced Bruchus spp. infestations. Because of the shorter growing period however, yields are also reduced. With regard to chemical control insecticides such as endosulfan can be applied in the field at early pod setting or harvested seeds can be fumigated with phosphine or methyl bromide. The annual routine fumigation conducted in Egypt decreased the infestation from more than 12% to less than 1%within 5 years.
- Screening of large numbers of germplasm for host plant resistance to B. incarnatus only revealed reduced infestation in the genotypes that are late in flowering and pod setting
Procedure followed at the centers in case of positive test
- Fumigation
References and further reading
http://www.inra.fr/hyppz/RAVAGEUR/6bruruf.htm
http://ressources.ciheam.org/om/pdf/a10/92605136.pdf
http://www.padil.gov.au/viewPestDiagnosticImages.aspx?id=394
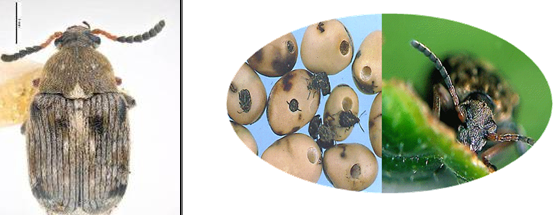 Bean Seed Beetle (photos: www.pgro.org) |
Cowpea seed beetle, Adzuki bean seed beetle
Scientific names
Callosobruchus maculatus (Fabricius), 1775, C. chinensis (L.), 1758.
Significance
The Callosobruchus beetles are principally a serious pest of stored products under hot dry conditions; complete destruction of grain and pulses may take place in a short time. In humid climates, the rates of increase of its competitors are so much greater that it has difficulty in establishing itself.
Symptoms (Damage)
The larval stage of the weevil tunnel and develop within the beans. They may consume nearly the entire bean contents. Pupation occurs in the beans and adults emerge through a round hole in the seed coat. Damage is a combination of the feeding and contamination.
Hosts
They attack several legumes in storage, chickpea (Cicer arietinum), arhar (Cajanus cajan), green gram (Vigna radiata), pea (Pisum sativum) and kidney bean (Phaseolus vulgaris) seeds. On lentil, C. chinensis is the most common.
Geographic distribution
Both species are widespread and found in all continents with subtropical or tropical conditions (USA, Mediterranean, India, and Australia).
Biology and transmission
The eggs are glued to the bean or the pod. On hatching the larvae bores into the seed where it makes a translucent 'window' in the seed before pupating. The larval and pupal stages are spent inside the bean. The adult emerges through the 'window' leaving a neat round hole. Infestations can begin in the field. Adults move to bean fields from trash beans left in sacks, harvesters, planters, or feed areas. The cowpea weevil readily attacks dried beans; thus this weevil can be a serious storage pest. Bean weevil infestations can also start in the field and may also originate from trash beans. As with the cowpea weevil, bean weevil will attack dried beans and can be a serious pest in stored beans. Broad bean weevil infestations also start in the field, but this pest is not a storage problem.
Detection/indexing method at ICARDA
- Visual inspection
Treatment/control
- Cleanliness in storage: Stores should be cleaned from all residues of grains, straw and flour and be de-infested by spraying walls with malathion, sacks, thresher and transportation vehicles also should be cleaned.
- Chemical control/protection: Seeds stored for food/feed can be fumigated with phospine (Phostoxin) available as 0.6 g pellets and 3 g tablets. The recommended dosage is 0.5-1 tablet/m3 with an exposure time of 2-4 and 3-5 days for pellets and tablets, respectively. The major advantages of Phostoxin are that it controls all insect stages, has no residues, does not affect flavor or germination and is easy to handle. Seeds stored for planting can be treated with insecticides such as Actellic at 4-10 ppm a.i. (0.5 g/kg seed) or malathion at 10 ppm a.i., which will protect the seeds for several months.
- Traditional methods of seed protection: Small quantities of seed stored for human consumption are often mixed with different vegetable oils or other substances. Mixing seeds with olive oil and salt (5 ml and 20 g/kg seed) or Neem seed oil (3 ml/kg seed) can provide adequate control for a period of 3-4 months.
Procedure followed in case of positive test
- Fumigation
References and further reading
http://www.icarda.org/Publications/Field_Guides/Lentil/Lentil.htm#Lent7.Html
http://www.infonet-biovision.org/default/ct/82/pests
http://agspsrv34.agric.wa.gov.au/Ento/pestweb/Query1_1.idc?ID=-1771861620
http://www.zin.ru/Animalia/coleoptera/rus/calmacdk.htm
http://www.uky.edu/~cfox/Students/Amarillo/Angela.html

Callosobruchus maculatus (photo: www.zin.ru/ ) |
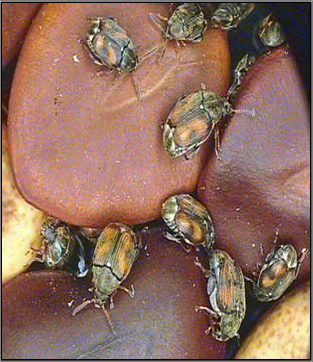
Callosobruchus maculatus (photo:www.uky.edu/) |

Callosobruchus chinensis |
Nematodes - faba bean
Contributors to this section: ICARDA, Syria (Siham Asaad, Abdulrahman Moukahal).
Stem and bulb nematode, Stem and bulb eelworm.
Scientific name
Ditylenchus dipsaci (Kühn, 1857) Filipjev, 1936.
Other scientific names
Tylenchus dipsaci (Kühn) Bastian, 1865. Ditylenchus phloxidis Kirjanova, 1951.
Ditylenchus fragariae Kirjanova, 1951.
Significance
D. dipsaci is one of the most devastating plant parasitic nematodes, especially in temperate regions. Without control, it can cause complete failure of host crops.
Symptoms
D. dipsaci causes swelling and deformation of stem tissue or lesions which turn reddish-brown then black, depending on cultivar and environmental factors. Newly formed pods take on a dark-brown appearance. The lesions envelop the stem and increase in length, often advancing to the edge of an internode. Leaf and petiole necrosis is also common under heavy infestations, but can be confused with symptoms induced by fungal leaf pathogens. Infected seeds are darker, distorted, and smaller in size and may have speckle-like spots on the surface. Heavy infestations often kill the main shoots, stimulating secondary tiller formation. The more severe symptoms are usually induced by the "giant race" on legumes.
Hosts
D. dipsaci is known to attack over 450 different plant species, including many weeds. However, it occurs in more than ten biological “races” some of which have a limited host-range. The race(s) that breed on rye, oats and onions seem to be polyphagous and can also infest several other crops, whereas those breeding on lucerne, Trifolium pratense and strawberries are virtually specific for their named hosts and seem to have relatively few alternative host plants. The tulip race will also infest Narcissus, whereas another race commonly found in Narcissus does not breed on tulip. It is known that some of the races can interbreed and that their progeny have different host preferences.
The principal hosts are faba beans, garlic, Hyacinthus orientalis, leeks, lucerne, maize, Narcissus pseudonarcissus, oats, onions, peas, Phlox drummondii, P. paniculata, potatoes, rye, strawberries, sugarbeet, tobacco, Trifolium pratense, T. repens, tulips. It has also been reported on carnations, celery, Hydrangea, lentils, rape, parsley, sunflowers, and wheat.
Geographic distribution
D. dipsaci occurs locally in most temperate areas of the world (Europe and the Mediterranean region, North and South America, northern and southern Africa, Asia and Oceania) but it does not seem able to establish itself in tropical regions except at higher altitudes that have a temperate climate. In most countries regulatory measures (e.g. certification schemes) are applied to minimize further spread of D. dipsaci.
Biology and transmission
In international trade D. dipsaci is liable to be carried on dry seeds and planting material of host plants. In the field the fourth-stage juvenile can withstand desiccation for many years, and although soil densities seem to decrease rapidly, the nematode can survive for years without a host plant. Nematode survival and damage are greater in heavy soils as compared to sandy soils. It can also survive on a number of weeds. Irrigation water and cultivation by contaminated farm tools and machinery are other sources of inoculum dissemination.
Detection/indexing method at ICARDA
- Nematode extraction test
Treatment/control
- Control by crop rotation is limited by the polyphagous habit of some races of D. dipsaci and by persistence of the nematode in clay soils. Chemical treatments of the soil are not an economic proposition for large areas. However, it may sometimes be worth treating small patches, after lifting and destroying the affected plants (bulbs) together with a margin of surrounding healthy ones, to eradicate a slight infestation before it spreads.
- Nematode-free (certified) seeds and planting material are most essential to prevent crop damage by D. dipsaci. Hot-water treatments with different temperature-time combinations, depending on type and state of seed material, are operational and efficient to control D. dipsaci. Systemic nematicides may be effective to some extent Ditylenchus dipsaci in controlling D.dipsaci in some ornamental crops. The use of tolerant or resistant cultivars can also reduce the damage.
- In laboratory experiments faba bean bulbs were heat treated in an attempt to control Ditylenchus dipsaci. A wide range of temperatures (43 to 50 deg C) and treatment times (30, 60, 120 min) was tested but none was successful. Chemical treatment, particularly preplanting treatment, was more successful. Calcium polysulphide (8%) and nemaphos (0.1 and 0.2%) were very effective.
Procedure followed at the centers in case of positive test
- Fumigation, rotation.
References and further reading
http://www.eppo.org/QUARANTINE/nematodes/Ditylenchus_dipsaci/DITYDI_ds.pdf
http://www.doacs.state.fl.us/pi/enpp/nema/nemacirc/nem187.pdf
http://www.ipmimages.org/browse/detail.cfm?imgnum=0162066
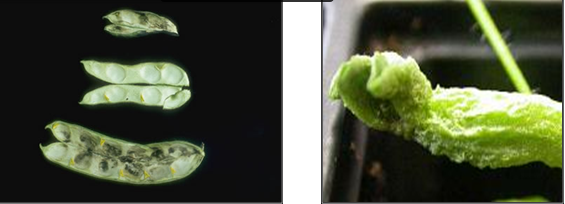 Stem and bulb nematode (photos:www.ipmimages.org/) |
Weeds - faba bean
Contributors to this section: ICARDA, Syria (Siham Asaad, Abdulrahman Moukahal).
|
Contents: |
Scientific names
Orobanche foetida Pers.
Orobanche crenata Forsk.
Orobanche ramosa L.
Significance
Yield reductions of 30 to 70% are not uncommon. International markets may reject broomrape-contaminated produce.
Symptoms
The appearance of the parasite itself is the most diagnostic feature of infestation. The parasite has erect, branched or unbranched aerial flowering shoots. The leaves are reduced to scales and are disposed alternatively. The shoots bear bisexual tubular flowers in a spike. The tiny seeds are produced in enormous numbers in capsules.
Hosts
Broomrapes usually parasitic only broadleaved plants but there are a few records of attacks on monocots (grasses, etc.). Branched broomrape attacks many crops and common weeds. Non-host crops can support infestations because of their weed component, and the produce of such crops may carry broomrape seeds. Some broomrape species have specialized biotypes or races. Plants of the same species attack different sets of hosts in different geographic areas. Susceptibility to attack also depends on the cultivar or genetic characteristics of a particular crop. The plants attacked by branched broomrape around the world include:
Crops: bean, broad bean, cabbage, capsicum (peppers), canola, carrot, cauliflower, celery, chickpea, clovers, eggplant (aubergine), flax, hemp (cannabis), hops, lentil, medics, onion, parsnip, paprika, pea, pyrethrum, sunflower, tobacco, tomato, potato.
Geographic distribution
Western and Central Asia (especially in Afghanistan, Syria, Egypt, India, Iran, Jordan, Lebanon, Morocco, Pakistan, Saudi Arabia, Australia, Europe (especially the Mediterranean), N. Africa, N. America.
Biology and transmission
Seeds germinate in moist soil at 18 to 23° C and require moist soil for one week to become established. The roots parasitic the roots of host plants for 40 to 50 days. Stems emerge in spring and are visible for only about 3 weeks from the end of September through to October. Flowering is rapid, within 6 to 9 days after stem emergence, with a similar period between the cessation of flowering and the ripening of fruit. An infestation may contain many plants that do not produce above-ground parts.
Dispersal from property to property and within properties in the infested area in South Australia appears to be mainly by carriage on vehicles and machinery. Seeds stick to equipment and animals that contact the plant and can also be dispersed by wind and water (including irrigation water/sprinklers), in animal manure, soil, fodder, on livestock and on footwear and clothing. Seeds attach to crop seeds and other produce. Seedlings imported from infested areas can carry seeds stuck to their leaves or in the soil. Seeds remain viable after passage through the digestive tracts of animals including cattle and goats.
Detection/indexing method in place at the CGIAR Center at ICARDA
- Filter wash test
Treatment/control
Hand weed shoots prior to their seed formation to reduce inoculum levels. This is recommended in minor infestations only.
- Sow the crop late to help plants escape the worst effects of infection.
- Plow deep to bury Orobanche seeds into deeper soil layers to reduce infestation.
- Use selective herbicides such as imazaquin (10-15 ml a.i. /ha).
- Use soil solarization to reduce soil-borne inoculum.
- Use resistant varieties, if available.
Procedure followed at the centers in case of positive test
- Mechanical cleaning, rotation
References and further reading
http://www.icarda.org/Publications/Field_Guides/Lentil/Lentil.htm#Lent7.Html
Branched Boomrape - Identification: State prohibited weed

Orobanche foetida (photo: ICARDA) |

Orobanche crenata (photo: ICARDA) |

Orobanche ramosa (photo: ICARDA) |

Orobanche egyptiaca (photo: ICARDA) |
Scientific names
Cuscuta campestris Yuncker
Cuscuta hyalina Roth.
Other scientific name
C. pentagona
Significance
The effect of field dodder on 12 legume crops was investigated in a greenhouse experiment. The legume crops showed great variations in response to field dodder parasitism. Based on the reduction in host dry weight (biological yield) caused by the parasite, faba bean lost >50% of its biological yield was considered highly susceptible.
Symptoms
The appearance of the parasite itself is the best diagnostic symptom. It has fine, yellow or orange, thread-like branches which grow and entwine around the stems and other aboveground parts of the host.
Dodder-infected areas appear as patches in the field and continue to enlarge during the growing season. In late spring and early summer, dodder produces a massed cluster of white flowers.
Infected host plants become weakened, decline in vigor and produce poor yields.
Several patches might coalesce to form large areas that can be easily recognized by the yellowish color of the parasite strands.
Hosts
C. campestrisis the most important Cuscuta species, attacking a wide (C. campestris) range of species, including vegetables, fruits, ornamentals and woody plants. It is reported as a weed in 25 crops in 55 countries.
Geographic distribution
Europe to North Africa and eastern Asia
Detection/indexing method at ICARDA
- Wash filter test
Treatment/control
- Cultural Practices: Dodder is best controlled through establishment prevention by using clean seed. Ornamental beds should be kept clean of seed-producing plants. Once dodder becomes established, eradication is almost impossible. Mechanical removal can be attempted, but once dodder has produced the haustoria it is near impossible to avoid damage to the host plant. If dodder has germinated and has attached to a host plant, removal of both is recommended to eradicate.
- Herbicide Use: Use of pre-emergent herbicides is the best chemical approach. These products must be applied prior to the germination of the seed as the small seedling root will drop off once the haustorium has formed. Dinitroanaline herbicides have been reported to be effective. Dichlobenil has also shown good results. Read all labels to ensure that the herbicides can be used around the host plants. Glyphosate has shown results as a postemergent herbicide, but its use is limited as it will also damage the host plant. Phenoxy herbicides have only shown limited control and again injury to the host plant can occur.
Procedure followed in case of positive test
- Mechanical cleaning. rotation
References and further reading
http://www.icarda.org/Publications/Field_Guides/Lentil/Lentil.htm#Lent7.Html
http://www.weedalert.com/weed_pages/wa_dodder.htm
 Dodder (photos:ICARDA) |



 stog-faba-bean
stog-faba-bean
