CGKB News and events stog-barley
Weeds - barley
Contributors to this section: ICARDA, Syria (Siham Asaad, Abdulrahman Moukahal).
|
Contents: |
Californian thistle, Canada thistle, Canadian thistle, Creeping thistle, Field thistle, Perennial thistle.
Scientific name
Cirsium arvense (L.) Scop.
Other scientic names
Carduus arvensis (L.) Robson, Cirsium arvense var. argenteum (Vest) Fiori, Cirsium arvense var. horridum Wimmer & Grab., Cirsium arvense var. integrifolium Wimmer & Grab., Cirsium arvense var. mite Wimmer & Grab., Cirsium arvense var. vestitum Wimmer & Grab., Cirsium incanum (Gmel.) Fisch., Cirsium setosum (Willd.) Bess. ex Bieb., Serratula arvensis L.
Importance
Can be serious.
Significance
Infestations reduce yields and contaminate crops. Yield losses of 5-15% are common, although locally, under severe infestations, losses can far exceed this amount.
Symptoms
Affected plants show irregular growth, atypical branching with little leaves, shortened internodes, hardening of the green inflorescence and reduced vigour, as well as either chlorosis or reddish-brown discoloration of the leaves and stems. Plants that reached flowering could also have multiple inflorescences. In the field the disease persists for years, increasing slowly around the original infection focus. Infected plants senesce prematurely, inhibiting both seed production and rhizome propagation, which markedly reduces the C. arvense population.
Hosts
Maize, sorghum, sugarcane, rice, Sudangrass, wheat, oats and barley.
Geographic distribution
It occurs throughout Europe, northern Africa, western and central Asia, northern India, Japan, China, northern North America, South Africa, New Zealand, Tasmania, and southeastern Australia, eastern Mediterranean and possibly northern Europe, western Asia, and northern Africa.
Biology and transmission
Germination may be affected by ecotype, temperature, day length, depth of seed burial, substrate stratification and seed freshness. Seeds from male plants are smaller and germination percentage is lower. Seeds germinate best in warm temperatures 20-40oC, with alternating light and dark periods. At lower temperatures germination is aided by high light intensity. Germination at higher temperatures can help ensure that maximum germination takes place during warmer periods of the year. Seeds are somewhat tolerant of heat and some were still viable after ten minutes at 102oC and two minutes at 262oC, although viability was decreased at these temperatures compared to unheated controls. The seeds germinate over a wide range of soil moisture.
Detection/indexing method in place at ICARDA
- Visual inspection.
Treatment/control
- Physical: C. arvense response to fire varies from positive to negative, depending on season of burn, soil moisture and location. Dormant season burning stimulates growth of native herbaceous species that compete with the weed. Growing season fire damages native species as well as C. arvense. Covering C. arvense with boards, sheet metal or tar paper can kill the plants.
- Chemical: Clopyralid plus 2, 4-D (sold under the trade name Curtail) provides the best and most consistent control in agricultural areas but may damage native forbs and shrubs. Fall application of clopyralid delayed shoot emergence by two weeks, and reduced shoot density the following summer. The impact of clopyralid increased with increased application rate, and application of 840 g/ha had the greatest impact. One autumn application with clopyralid at 560 g/ha prevented almost all C. arvense shoot emergence the following spring.
Procedure followed at the CGIAR Centres in case of positive test
- Cleaning seed lot.
 |
 |
|
|
Californian thistle (photos: ICARDA) |
||
References and further reading
http://issg.org/database/species/reference_files/cirarv/cirarvman.pdf
http://issg.org/database/species/ecology.asp?si=413&fr=1&sts=sss⟨=EN
Asiatic witchweed, Buri, Common mealie witchweed, Isona weed, Matabele flower, Mealie poison, Mealie witchweed, Red witchweed, Scarlet lobelia, Striga, Witchweed.
Scientific names
Other scientific name
S. lutea Lour.
Importance
Minor.
Significance
Infestations reduce yields and contaminate crops. Yield losses of 5-15% are common, although locally, under severe infestations, losses can far exceed this amount.
Symptoms
Severe attack produces leaf wilting and chlorosis. Infected plants may be stunted and die prior to seed set.
Hosts
Pearl millet, maize, sorghum, sugarcane, rice, Sudangrass, wheat, oats and barley.
Geographic distribution
Africa, Asia, Europe, Australasia-Pacific Region and North America.
Biology and transmission
S. asiatica seeds are dispersed with wind, water, soil movement, human activity and by clinging to the feet, fur or feathers of animals, farm machinery, tools, shoes and clothing. Seeds require an after-ripening period of 6 weeks under warm conditions to 40 weeks under freezing conditions. Dormant seeds survive freezing for at least 49 days and can remain viable under field conditions for up to 14 years or more. Germination is complex and requires about a 1-3 week "conditioning" period at a suitable temperature regime under moist conditions, followed by a chemical signal from a nearby root of a host plant. Proximity of host root to seed must be within a few millimetres. Under these conditions, seeds germinate within 24 hours. After 3 weeks of conditioning without a chemical signal, germination ability of seeds decrease, and some seed may pass into a secondary dormancy. Light exposure or wet soils inhibit germination. Irregular or light rainfall appears to promote seed germination and plant vigour. High soil nitrogen reduces damage to host plants. Flowers develop about three weeks after emergence. Viable seed is produced within two weeks of flowering. A minimum of ~ 60 days is required from seed germination to seed production.
Detection/indexing method in place at ICARDA
- Not important.
Treatment/control
Integrated management: The California Deptartment of Food and Agriculture [CDFA (2006)] suggests that light infestations can usually be controlled by hand pulling before seed is produced. For heavier infestations, an integrated management plan is required. Options include :
- Growing trap-crops (those that stimulate S. asiatica seed germination but do not host the parasite) such as cotton or catch-crops (susceptible crops that are harvested before S. asiatica seed is produced) for three or more years.
- Allowing land to lay fallow for several years; injecting the soil with ethylene (a germination stimulant).
- Enhancing soil nitrogen fertility.
- Growing the most tolerant cereal varieties.
- Utilizing herbicides known to prevent S. asiatica emergence or seed production.
Procedure followed at the CGIAR Centres in case of positive test
- Not applicable.
References and further reading
http://issg.org/database/species/ecology.asp?si=968&fr=1&sts=⟨=EN
http://www.tifton.uga.edu/fat/PlantParasitesPM.htm
http://www.invasive.org/browse/detail.cfm?imgnum=1148083
Seed Health General Publication Published by the Centre or CGIAR
 |
 |
|
|
Weed attaches to barley plant and witchweed (photos: www.tifton.uga.edu) |
||
Nematodes - barley
Contributors to this section: ICARDA, Syria (Siham Asaad, Abdulrahman Moukahal).
Seed Gall Nematode
Scientific name
Anguina tritici (Steinbuch, 1799) Filipjev, 1936
Importance
Seedborne.
Significance
Nematode damage is negligible in countries adopting modern mechanical and cleaning procedures to separate the nematode galls from visible wheat seeds. The use of high quality seeds has nearly eradicated this nematode from developed countries. However, the nematodes cause severe crop losses to rye (35-65%) and wheat in third world countries, where poor agricultural practices, monoculture and the use of poor quality seeds are widespread. In spite of the insignificant damage caused by the nematode in modern agricultural production systems of developed countries, their ability to export grains to the international markets is severely hampered if historical records still record the presence of this pest in grain production areas due to the quarantine measures imposed by many countries because of this pest.
Symptoms
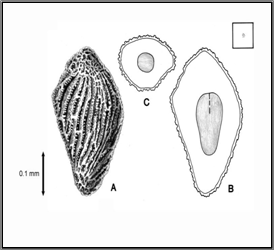
Distorted leaves and stems are evident prior to heading. As diseased plants approach maturity, galls are formed in the florets, replacing the kernels. The galls are similar in shape to the seed they replace and are dark brown in colour. Large numbers of motile larvae are present within the galls and become active after the galls have been moistened. These nematodes can act as vectors of Corynebacteriurn tritici.

Healthy seed Seed Galls

Diseased spike
Source: www.forestryimages.org
Hosts
Emmer (Triticum monococcum), rye (Secale cereale), spelt (T. spelta) and wheat (T. aestivum). Barley (Hordeum vulgare) is a very poor host. There is no evidence that this nematode reproduces on oats (Avena sativa) and other grasses.
Geographic distribution
USA, Australia, Austria, Brazil, China, Egypt, England, Ethiopia, France, Germany, India, Italy, Hungary, the Netherlands, New Zealand, Pakistan, Romania, Sweden, Switzerland, Syria, Turkestan, Russian Federation, Serbia, Montenegro, Kosovo, Macedonia FYR, Boznia & Herzegovina, Croatia, and Slovenia.
Biology and transmission
These nematodes migrate as J2s in water films to plant leaves where they feed as ectoparasites at the tips, causing distortion of the leaves. Once the plant starts to flower the J2 penetrates the floral primordia and starts to feed on the developing seed. Once in the seed, the nematode undergoes its moults, continues to feed and eventually kills the seed to form a blackened "cockle" (Seed Gall). The adults sexually reproduce, the eggs hatch as J1 and then quickly moult into a J2 survival stage. The environmentally resistant J2 desiccates with the Seed Gall and overwinters. The nematodes in the Sseed Gall can survive for 30 years if kept in a dry location. When proper moisture and temperature conditions arise, the cryptobiotic J2 becomes active and starts the life cycle over again.
Detection/indexing method in place at ICARDA
- Not significant.
Treatment/control
- Crop rotation for one to two years to a non-host eliminates A. tritici from the soil.
- Seed can be cleaned by placing it in a 20% brine solution; galls float to the surface where they can be separated. The seed is then rinsed and dried (heat treated).
- Dry heat for sterilizing containers: 50oC for 30 minutes is effective.
- Flaming of metallic equipment - shovels, sampling tools, etc.
- Soil solarization. Consider the energy cost of heating soil.
- Mechanical separation is also effective in removing galls from seed.
Procedure followed at the CGIAR Centres in case of positive test
- Not applicable.
References and further reading
http://nematode.unl.edu/pest67.htm
http://www.doacs.state.fl.us/pi/enpp/nema/nemacirc/nem186.pdf
http://plpnemweb.ucdavis.edu/NEMAPLEX/Taxadata/G006S4.htm
http://wheatdoctor.cimmyt.org/index.php?option=com_content&task=view&id=75&Itemid=43
http://www.forestryimages.org/search/action.cfm?q=mcclure



 stog-barley
stog-barley
