CGKB News and events stog-groundnut
Fungi (groundnut)
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
|
Contents: |
Scientific name
Verticillium dahliae Kleb. and Verticillium albo-atrum Reinke & Berthie
Other scientific names
Verticillium albo-atrum var. chlamydosporale, Verticillium albo-atrum var. dahliae, Verticillium albo-atrum var. medium, Verticillium dahliae f. chlamydosporale, Verticillium dahliae f. medium, Verticillium ovatum, Verticillium tracheiphilum.
Importance to CGIAR centers
High
Significance
Verticilliumdahliae affects many important crops including peanut and causes losses of economic significance in many countries.
Symptoms
Early symptoms usually appear at the flowering stage and include marginal chlorosis of the leaves, loss of leaf turgidity and leaf curling. Leaf symptoms are generally yellowing and leaflet necrosis, followed by wilting and defoliation. The roots of the infected plants have brown discoloration of the vascular tissues. Occasionally plants die, and the roots of the dead plants are severely rotted (Subrahmanyam et al. 1992).
Hosts
Verticilliumdahliae has a very wide host range among economically important crops such as Gossypium (cotton), Solanum tuberosum (potato), Solanum melongena (aubergine), Capsicum annuum (bell pepper), Olea europaea subsp. europaea (olive), Brassica napus var. napus (rape), Fragaria ananassa (strawberry), Humulus lupulus (hop), Lycopersicon esculentum (tomato), Medicago sativa (lucerne), Mentha (mints),Arachis hypogaea (groundnut), Armoracia rusticana (horseradish), Brassica oleracea var. gemmifera (Brussels sprouts), Pistacia vera (pistachio), Prunus (stone fruit) and Vitis vinifera (grapevine).
Geographic distribution
Verticilliumdahliae is worldwide in distribution, including Asia, Africa, Europe, USA and Australia.
Biology and transmission
Verticilliumdahliae is moderately to fast-growing fungus with little to moderate aerial mycelium and a regular margin, turning black from the centre after a week due to production of microsclerotia. Conidiophores are verticillate and conidiogenous cells subtended in whorls (2-3 per node), and are erect and hyaline. Conidia are ellipsoidal, hyaline, mostly one-celled and produced at the tips of narrow, pointed sterigmata. Conidia are 2.5-6 ´ 1.5-3.0 µm in size. Conidia are produced in succession to form moist spore balls at the tips of conidiogenous cells, giving characteristic appearance to conidiophore in culture. Microsclerotia are of irregular shape and size (50-200 ´ 15-100 µm), dark brown to black and globose. The fungus survives in soil as microsclerotia which germinate in response to root exudates. The hyphae or germinating conidia penetrate the cortex of young roots and the fungus grows into the stele. In the xylem vessels the pathogen spreads by mycelial growth, and also by the production of conidia which get into transpiration stream. Microsclerotia are formed in senescing diseased tissues. The pathogen is disseminated throughout the field soil by farm equipment, wind and water movement and by infected seed.
Detection/indexing methods used in CGIAR at ICRISAT
- Pre export field inspection and blotter test are used.
Treatment/control
- Not available.
Procedures followed in case of positive test at ICRISAT
- Incineration of the infected plants and rejection of the infected seed samples.
EPPO protocols
EPPO A2 list: No. 85
Detection. Use of DNA hybridization probes (Robb et al. 1990) and ELISA test for V. albo-atrum are in use in France for testing certified pelargonium (OEPP/EPPO 1992).
Phytosanitary risk. EPPO has listed hop-infecting strains of V. albo-atrum and V. dahliae as A2 quarantine pests (OEPP/EPPO 1982), but no other regional plant protection organization has done so. Regulatory control may remain appropriate, but may take on the character of a certification scheme for planting material.
Phytosanitary Measures. EPPO recommends (OEPP/EPPO 1990) that hop planting material should come from a field where verticillium wilt has not occurred in the last 5 years and that consignments and their mother plants should have been found free from the disease in the last growing season. Such measures are as relevant in a national certification scheme as for international phytosanitary certification.
References and further reading
OEPP/EPPO. 1982. Data sheets on quarantine organisms No. 85, Hop-infecting strains of Verticillium albo-atrum and V. dahliae. Bulletin, OEPP/EPPO Bulletin12 (1).
OEPP/EPPO. 1990. Specific quarantine requirements. EPPO Technical DocumentsNo. 1008.
OEPP/EPPO. 1992. Certification schemes No. 3. Pathogen-tested material of pelargonium. OEPP/EPPO Bulletin22: 285-296.
Robb J, Hu X., Schmidt J, Nazar R. 1990. DNA hybridization probes for the identification and quantification of V. dahliae and V. albo-atrum. In: Abstracts of the 5th International Verticillium Symposium, Leningrad, USSR, p. 97.
Subrahmanyam P, Wongkaew S, Reddy DVR, Demski JW, McDonald D, Sharma SB, Smith DH. 1992. Field diagnosis of groundnut diseases. Information bulletin no. 36, Patancheru, AP, 502 324, India: International Crops Research Institute for the Semi Arid Tropics. 84pp.
 Verticillium wilt (Verticillium dahliae) of groundnut: marginal chlorosis, leaf curling, tiger striping and yellowing of leaves(photo:ICRISAT) |
Scientific name
Colletotrichum dematium (Pers.) Grove.
Other scientific names
Colletotrichum bakeri, Colletotrichum brassicae, Colletotrichum lysimachiae, Colletotrichum pucciniophilum, Colletotrichum sanguisorbae, Colletotrichum volutella, Dinemasporium dianthi, Ellisiellina volutella, Sphaeria dematium, Vermicularia bakeri, Vermicularia dematium, Vermicularia dianthi, Vermicularia echinata, Vermicularia lagunensis, Vermicularia lysimachiae, Vermicularia volutella.
Importance to CGIAR centers
Low
Significance
Anthracnose in peanut is of minor importance.
Symptoms
Symptoms appear as wedge-shaped lesions on the leaflet tips. Lesions may also develop on the leaflet margins leading to marginal blight. The periphery of the advancing margins of the lesion is surrounded by the yellow zone. The necrotic tissue becomes dark brown and tends to fragment along the leaflet margins. The disease may also extend to stipules and stems. Fruiting bodies (acervuli) are visible through a hand lens, and are abundant on infected leaf tissue (Subrahmanyam et al. 1992).
Hosts
Allium cepa (onion), Allium porrum (leek), Allium sativum (garlic), Arachis hypogaea (groundnut), Beta vulgaris (beetroot), Helianthus annuus (sunflower), Piper betle (betel pepper), Vicia faba (broad bean), Voandzeia subterranea (bambara groundnut), Abelmoschus esculentus (okra), Allium (onions, garlic, leek, etc.), Cicer arietinum (chickpea), Capsicum annuum (bell pepper), Crotalaria juncea (sunn hemp), Glycine max (soyabean), Lablab purpureus (hyachinth bean), Lycopersicon esculentum (tomato), Vigna radiata (bean, mung), Vigna mungo (black gram), Spinacia oleracea (spinach).
Geographic distribution
Colletotrichum dematium is worldwide in distribution, especially India, Niger, Nigeria, Sudan, Senegal, Taiwan, Tanzania, Thailand, Uganda and USA.
Biology and transmission
Mycelium of C.dematium is hyaline, it produces circular, errumpt, dark brown to black acervuli. These acervuli are scattered on the infected pods or aggregated or in groups. Acervuli exude spores in pale to smoke-gray masses. Numerous thick, black, erect setae are interspersed within the acervuli. Conidia are hyaline, 1-celled and 2.5-4.0 ´ 15-32 mm in size. They are fusoid and bluntly tapered at both ends (Ahmed and Ravinder Reddy 1993).
Detection/indexing methods used in CGIAR at ICRISAT
- Pre export field inspection and blotter test are used.
Treatment/control
- Not available.
Procedures followed in case of positive test at ICRISAT
- Rejection of seed samples in case of positive test.
References and further reading
Ahmed KM, Ravinder Reddy Ch.1993. A Pictorial guide to the identification of seed borne fungi of sorghum, pearl millet, finger millet, chickpea, pigeonpea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the semi-Arid Tropics. 200 pp.
Subrahmanyam P, Wongkaew S, Reddy DVR, Demski JW, McDonald D, Sharma SB, Smith DH. 1992. Field diagnosis of groundnut diseases. Information bulletin no. 36, Patancheru, AP, 502 324, India: International Crops Research Institute for the Semi Arid Tropics. 84pp.
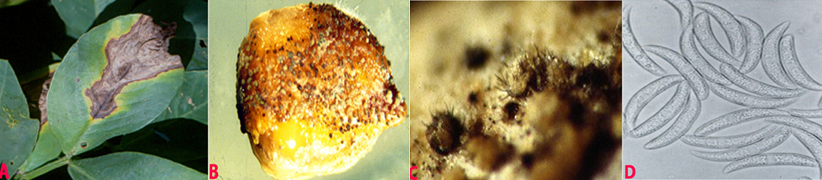 Anthracnose (Colletotrichum dematium) of groundnut: (A)wedge-shaped lesions on the leaflet; (B)fungal growth on seed; (C)acervuli with setae on seed and (D)conidia (photos:ICRISAT) |
Scientific names
Rhizoctonia bataticola (Tassi) E.J. Butler, Macrophomina phaseolina (Tassi) Goid.
Other scientific names
Botryodiplodia phaseoli, Dothiorella cajani, Dothiorella phaseoli, Dothiorella philippinensis, Fusicoccum cajani, Macrophoma cajani, Macrophoma corchori, Macrophoma phaseoli, Macrophoma phaseolina, Macrophoma sesami, Macrophomina philippinensis, Rhizoctonia lamellifera, Sclerotium bataticola, Tiarosporella phaseoli, Tiarosporella phaseolina.
Importance
High
Significance
Charcoal rot is economically important across a broad range of crops throughout the world, particularly in regions that experience hot, dry conditions during the growing period. Yield losses in groundnut of 100, 94 and 63% have been reported when disease appeared at the pre-emergence, pre-pod and pod-filling stages, respectively (Sharma and Bhowmik 1986).
Symptoms
Water-soaked lesions appear on the hypocotyl near the soil surface. The lesions enlarge, become dull brown, girdle the hypocotyle, and kill the plants. Lesions on the roots appear water-soaked at first, but infected tissues eventually have a dull, light-brown appearance. Later, affected areas become covered with sclerotia. Roots become rotten and blackened with shredding of the taproot. The dead tissues rot and turn black, as sclerotia of the fungus develop profusely. Infected pegs and pods also rot and become covered with sclerotia.
Hosts
Many crop plants including Allium cepa (onion), Allium sativum (garlic), Arachis hypogaea (groundnut), Beta vulgaris var. saccharifera (sugarbeet), Brassica oleracea var. botrytis (cauliflower), Cajanus cajan (pigeon pea), Carthamus tinctorius (safflower), Cicer arietinum (chickpea), Cyamopsis tetragonoloba (clusterbean), Coriandrum sativum (coriander), Capsicum annuum (bell pepper), Cucumis melo (melon), Cucumis sativus (cucumber), Curcuma longa (turmeric), Crocus sativus (saffron), Glycine max (soyabean), Helianthus annuus (sunflower), Nicotiana tabacum (tobacco), Oryza sativa (rice), Pennisetum glaucum (pearl millet), Vigna radiata (bean, mung), Vigna mungo (black gram), Phaseolus vulgaris (common bean), Sorghum bicolor (common sorghum), Vigna unguiculata (cowpea) and Zea mays (maize).
Geographic distribution
Rhizoctonia bataticola is world wide in distribution.
Biology and transmission
Sclerotia are black, smooth, hard and 0.1-1 mm diameter, and occur within roots, stems, leaves and fruits. Conidiomata are pycnidial, dark-brown, and either solitary or gregarious on leaves and stems; they are immersed, becoming erumpent, 100-200 µm diameter, opening by an apical ostiole; the conidiomatal wall is multicellular with heavily pigmented, thick-walled cells on the outermost side. Conidiophores are hyaline, short and obpyriform to cylindrical, 5-13 ´ 4-6 µm. Conidia are hyaline, ellipsoid to obovoid, 14-30 ´ 5-10 µm (Ahmed and Ravinder Reddy 1993). R. bataticola or M. phaseolina was detected in the seed coat, cotyledons and embryo of groundnut (Charabarty et al. 2005). It survives under different temperatures from -18°C to 20 °C temperatures (Singh et al. 2003).
Detection/indexing methods at ICRISAT
- Pre export field inspection and blotter test.
Treatment/control
- Seed treatment with a mixture of carbendazim and thiram (1:1) at 2 g a.i. kg-1 seed.
Procedures followed in case of positive test at ICRISAT
- If the seed colonization is <20% seed treatment with a mixture of carbendazim and thiram (1:1) at 2 g a.i. kg-1 is used, and seed samples having >20% colonization are rejected.
References and further reading
Ahmed KM, Ravinder Reddy Ch.1993. A Pictorial guide to the identification of seed borne fungi of sorghum, pearl millet, finger millet, chickpea, pigeonpea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the semi-Arid Tropics. 200 pp.
Chakrabarty SK, Girish AG, Anitha K, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Detection, seedborne nature, disease transmission and eradication of seedborne infection by Rhizoctonia bataticola (Taub.) Butler in groundnut, Indian Journal of Plant Protection 33: 85-89.
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Sharma RC, Bhowmik TP. 1986. Estimation of yield losses in groundnut due to Macrophomina phaseolina (Tassi) Goid. Indian Journal of Plant Pathology 4:108-112.
Singh SD, Girish AG, Kameswar Rao N, Bramel PJ, Subhash Chandra. 2003. Survival of Rhizoctonia bataticola in groundnut seed under different storage conditions. Seed Science and technology Journal 31: 169-175.
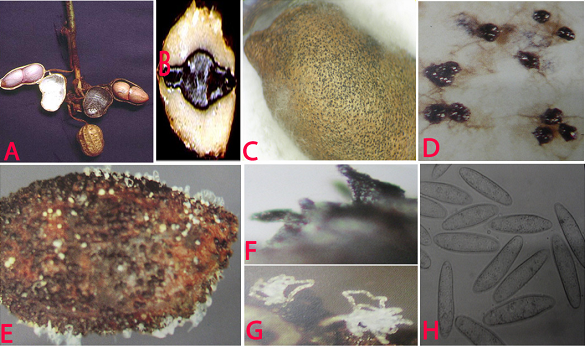 Charcoal rot (Rhizoctonia bataticola) of groundnut: (A)water soaked necrotic lesions on pods; (B)infected cotyledon; (C)infected seed with sclerotia; (D)sclerotia; (E)pycnidia and conidial ooze on seed; (F)pycnidia; (G)conidial ooze and (H)conidia of Macrophomina phaseolina (photos:ICRISAT) |
Nematodes - groundnut
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
|
Contents: |
Potato tuber nematode, Potato rot nematode
Scientific name
Ditylenchus destructor Thorne.
Importance
Medium
Significance
It has been a problem in all the groundnut-producing areas of South Africa (Jones and De Waele 1988). It is suspected that the population in South Africa may be a separate ecotype or pathotype and may be confined to groundnuts.
Symptoms
The most common symptoms include stunting and chlorosis of affected plants. Hulls of groundnuts show black discoloration which appears first along the longitudinal veins. The kernels are shrunken. The infected testae are brown to black and the embryo shows a brown discoloration (Jones and De Waele 1988).
Host
Potatoes (Solanum tuberosum) are the main host of D. destructor, but the nematode can also occasionally be found on bulbous Trifolium spp. (clover), Daucus carota (carrots), Arachis hypogaea (groundnut) and Allium sativum (garlic). Overall, some 70 crops and weeds and a similar number of fungus species have been recorded as hosts (Thorne 1961).
Geographical distribution
Ditylenchus destructor is worldwide in distribution.
Biology and transmission
Adults of D. destructor are minute worm-like animals, 0.8-1.4 mm in length and 23-47 μm in diameter. Considerable morphometric variation occurs in adults according to their host and/or age. Males and females are similar in general appearance. In females, the ost-vulval sac extends about three-quarters of the distance to the anus, and the tail has a narrow rounded terminus. Males have ventrally curved, anteriorly expanded spicules. There are four juvenile stages (the first preceding hatching of the egg), superficially similar to adults, but differing in size and in lacking developed reproductive organs. Unlike the closely related species D. dipsaci, D. destructor is unable to withstand excessive desiccation, and for this reason it is usually more important in cool and moist soils. Without a resistant resting stage, the species overwinters in soil as adults or larvae and may even multiply by feeding on alternative weed hosts (e.g. Mentha arvensis, Sonchus arvensis) and on fungal mycelium. It may also possibly overwinter as eggs. These hatch in the spring and larvae are immediately able to parasitize hosts. Egg hatch at 28°C begins 2 days after egg laying, with an average interval of 4.4 days between egg laying and hatch, and development from egg to adult takes between 6 and 7 days (Hooper 1973).
Detection/indexing methods at ICRISAT
- Pre export field inspection and seed washing to detect the nematodes.
Treatment/control
- Seed dressing of groundnut prior to planting with thiram or benomyl wettable powder gave very good control (Fujimura et al. 1989).
Procedures followed in case of positive test at ICRISAT
- Incineration of the infested crop and rejection of the seed samples are used.
EPPO protocols
Ditylenchus destructor was considered to be an EPPO A2 quarantine pest (OEPP/EPPO 1978) but was deleted from the quarantine list in 1984 because of its minor importance and very wide distribution throughout the EPPO region, in particular in those areas where it would be likely to cause crop damage.
References and further reading
Fujimura T, Ichita T, Kimura T. 1989. Occurrence of potato-rot nematode, Ditylenchus destructor Thorne, in garlic and control. 1. Evaluation of treatments applied before planting and after harvest for control. Japanese Journal of Nematology18: 22-29.
Hooper DJ. 1973. Ditylenchus destructor. CIH Descriptions of Plant-parasitic Nematodes No. 21. CAB International, Wallingford, UK.
Jones BL, De Waele D. 1988. First report of Ditylenchus destructor in pods and seeds of peanut. Plant Disease 72: 453.
OEPP/EPPO. 1978. Data sheets on quarantine organisms No. 123, Ditylenchus destructor. Bulletin OEPP/EPPO Bulletin 8(2).
Thorne G. 1961. Principles of nematology, 533 pp. McGraw-Hill Book Co. Inc., New York, USA.
Scientific name
Aphelenchoides arachidis Bos.
Importance
High
Significance
Nematode infection does not suppress yield, but causes serious qualitative damage and predisposes seeds to fungal infection by Fusarium spp., Macrophomina phaseolina, Rhizoctonia solani and Sclerotium rolfsii. These infected seeds are under graded and unmarketable (Bridge and Hunt 1985; Minton and Baujard 1990; Stokes 1980).
Symptoms
Aphelenchoides arachidis occurs as an endoparasite on the tissues of the pods, testas, roots and hypocotyls. Testas infected with A. arachidis are thicker and more uneven than normal testas. Heavily infected seeds had translucent testas shortly after removal of the fully mature pods. These seeds are also a lighter brown and have darker vascular strands within testas (Stokes 1980).
Host
Arachis hypogaea (peanut) is the principal host; other agronomic crops and weeds can be infected without showing any symptoms or damage. These hosts include Zea mays (maize), Oryza sativa (rice), Saccharum offcinarum (sugarcane) and unidentified grasses (CABI 2001).
Geographical distribution
This nematode has been reported only from Nigeria.
Biology and transmission
Females of Aphelenchoides arachidis are characterized by a lateral field marked by two incisures, a stylet of 11-12 µm long with distinct knobs, a postvulval uterine sac extending for half the vulva-anus distance, and a sub-cylindroid tail with a bluntly rounded terminus provided by a mucro. The nematode develops and reproduces in the seed coat (testa), and also in the pod, root and hypocotyl tissues causing discoloration, necrosis and brown stripes within the testas. The nematode-infected seeds appear shrunken and dark. This pest can survive in low numbers in pods and seeds (Bridge and Hunt 1985). The nematode is disseminated by infected peanut hulls and seeds. Because of its limited distribution (Nigeria), A. arachidis has not caused major economic losses, but this pest can become a major economic pest if introduced in large peanut-producing areas (Minton and Baujard 1990).
Detection/indexing methods at ICRISAT
- Seed washing test is used to detect the nematode.
Treatment/control
- Hot water treatment at 60°C for 5 min. eliminates the nematode (Stokes 1980).
Procedures followed in case of positive test at ICRISAT
- Incineration of the plants and rejection of the seed samples in case of positive test.
References and further reading
Bridge J, Hunt DJ. 1985. Aphelenchoides arachidis. CIH description of plant parasitic nematodes Set 8, No. 116, pp. 3. St. Albans, UK: Commonwealth Institute of Helminthology.
CAB International. 2001. Aphelenchoides arachidis In: Crop protection compendium, global module, 3rdedition. Wallingford, UK: CAB International.
Minton N A, Baujard P. 1990. Nematode parasites of peanuts. Pp. 285-320 in (M. Luc, R. A. Sikora, and J. Bridge eds) Plant parasitic nematodes in tropical and subtropical agriculture. Wallingford, UK: CAB International.
Stokes DE. 1980. The effects of the testa nematode, Aphelenchoides arachidis, on peanuts. Nematology Circular No. 68, Florida Department of Agriculture and Consumers Services, Division of Plant Industry, Gainesville, FL, USA.
Best practices for the safe transfer of groundnut germplasm
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
Seed health testing protocol at ICRISAT-PQL for import
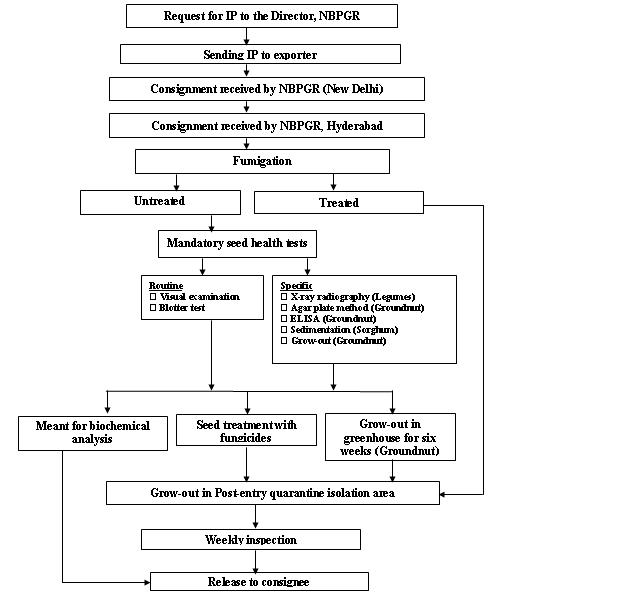
Seed health testing protocol at ICRISAT-PQL for export
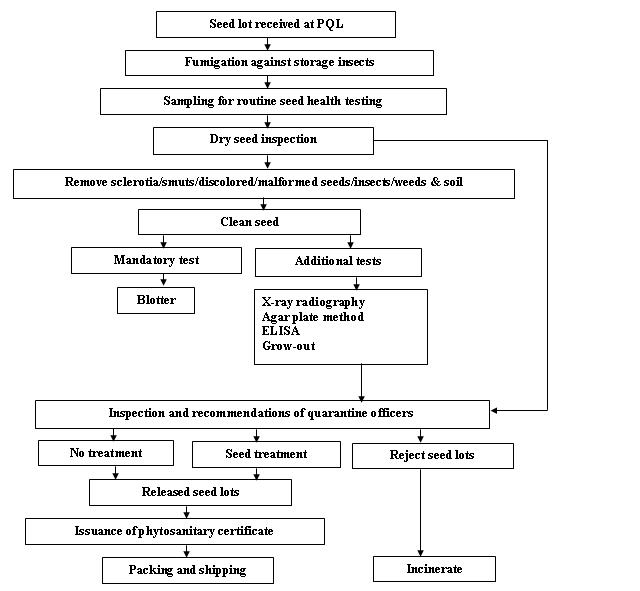
References and further reading
Chakrabarty, SK, K. Anitha, AG Girish, B Sarath Babu, RDVJ Prasad Rao, KS Varaprasad, PK Khetarpal and RP Thakur. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin 69. Rajendranagar 500 030, Andra Pradesh, India. National Bureau of Plant Genetic Resources, Pantacharu 502 324, Andra Pradesh, India. International Crops Research Institute for the Semi-Arid Tropics (ICRISAT).
Viruses - groundnut
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
|
Contents: |
Scientific name
Peanut mottle virus (PMoV)
Other scientific names
Peanut green mosaic virus, Peanut chlorotic mottle virus
Importance
High
Significance
PMoV causes substantial yield losses in many parts of the world. In some South East Asian countries losses up to 30-48% have been reported, and in the Indian Sub-Continent the virus is a potential threat to groundnut production (Reddy 1991; Prasada Rao et al. 1996).
Symptoms
Leaf symptoms on groundnuts include mild dark-green mosaic or mottle; leaflet margins can be crinkled and interveinal tissue depressed. Occasional leaf necrosis and deformation, chlorotic spots and stunting are also observed. Infected seeds are often malformed and discolored (Abdelsalam et al. 1987).
Hosts
Arachis chacoense (wild groundnut), Phaseolus vulgaris (common bean), Lupinus angustifolius (lupins), Vigna radiata (mung bean), Pisum sativum (pea), Glycine max (soybean) and forage legumes.
Geographic distribution
PMoV is worldwide in distribution including East Africa, South East Asia, India, Philippines, Taiwan, Malaysia, South America and South East USA (Reddy 1991).
Biology and transmission
PMoV has flexuous, filamentous, non-enveloped particles ranging from 723 to 763 nm in length and 12 nm in diameter (Pietersen and Garnett 1992). Infected groundnuts are considered to be the primary source of PMoV (Prasada Rao et al. 1993) and other nearby leguminous crops become infected from this crop. In addition to being mechanically transmissible, PMoV is also transmitted in a non-persistent manner by several species of aphid, including Aphis craccivora, Aphis gossypii, Hyperomyzus lactucae, Myzus persicae, Rhopalosiphum maidis and Rhopalosiphum padi (Pietersen and Garnett 1992). PMoV is seedborne up to 20% in groundnuts (Bashir et al. 2000). Adams and Kuhn (1977) reported that seed transmission is due to the presence of the virus in the embryo.
Detection/indexing methods at ICRISAT
- Pre export field inspection and ELISA (Sudarshan and Reddy 1989) are used.
Treatment/control
- Not known.
Procedures followed in case of positive test at ICRISAT
- Rejection of seed samples in case of positive test.
References and further reading
Abdelsalam AM, Khalil EM, Fahim MM, Ghanem GA. 1987. The effect of peanut mottle virus infection on growth and yield of peanuts. Egyptian Journal of Phytopathology 19:127-132.
Adams DB, Kuhn CW. 1977. Seed transmission of peanut mottle virus in peanuts. Phytopathology 67:1126-1129.
Bashir M, Ahmad Z, Murata N. 2000. Seed-borne viruses: detection, identification and control, Pakistan Agricultural Research Council, National Agricultural Research Center, Park Road, Islamabad, Pakistan, 156pp.
Pietersen G, Garnett HM 1992. Some properties of a peanut mottle virus (PMoV) isolate from soybeans in South Africa. Phytophylactica 24:211-215.
Prasada Rao RDVJ, Ribeiro GP, Pittman R, Reddy DVR, Demski JW. 1993. Reaction of Arachis germplasm to peanut stripe, peanut mottle and tomato spotted wilt viruses. Peanut Science 20:115-118.
Prasada Rao RDVJ, Chakrabarthy SK, Reddy AS, Girish AG, Mehan VK. 1996. Interception of peanut stripe virus in groundnut germplasm imported from China. Indian Journal of Plant Protection 24:143-145.
Reddy DVR. 1991. Crop profile. Groundnut viruses and virus diseases: distribution, identification and control. Review of Plant Pathology 70: 665-678.
Sudarshana MR, Reddy DVR. 1989. Penicillinase-based enzyme-linked immunosorbent assay for the detection of plant viruses. Journal of Virological Methods 26:45-52.
 Peanut mottle (Peanut mottle virus) of groundnut: symptoms of irregular dark green islands and interveinal depression (photo:ICRISAT) |
Scientific name
Peanut stripe virus (PStV).
Importance
Low
Significance
PStV has been reported to cause about 50% incidence in China ( Xu et al. 1994). In Gujarat, India, the disease incidence has been recorded up to 40% (Varma et al. 1994). In South East Asia, high incidences of up to 38% have been reported in Indonesia (Middleton and Saleh 1988) and the Philippines (Adalla and Natural 1988). PStV infection has a highly variable effect on groundnut yield, depending on the test conditions, cultivar and the virus isolate.
Symptoms
Symptoms on groundnut plants vary, depending on virus isolate and groundnut cultivar. For most isolates, the initial symptoms appear as chlorotic flecks or rings on young quadrifoliates. The plants are slightly stunted. Subsequently, the older leaves show symptoms which are more specific to the isolate: mild mottle, blotch, stripe, chlorotic ring mottle, chlorotic line pattern, oak leaf pattern or necrosis (Wongkaew and Dollet 1990). The symptoms normally persist throughout plant development. The 'stripe' [V-shaped or herringbone pattern] and 'necrotic' isolates, which are seen less often, can severely stunt the plants if they infect them early.
Hosts
Arachis hypogaea (groundnut). Glycine max (soyabean), Lupinus albus (lupine), Medicago sativa (lucerne), Vigna radiata (mung bean), Sesamum indicum (sesame), Vigna unguiculata (cowpea). Cassia occidentalis (coffee senna), Cassia tora (foetid cassia), Centrosema pubescens (Centro), Calopogonium caeruleum, Crotalaria pallida (smooth crotalaria), Desmodium (tick clovers), Indigofera (indigo), Pueraria phaseoloides (tropical kudzu), Cassia obtusifolia (sicklepod) and Stylosanthes biflora (Pencil flower).
Geographic distribution
PStV is widespread in groundnut-growing areas throughout east and south Asia. It was first detected in India in 1987 (Demski et al. 1993. It also occurs in all groundnut growing areas of Indonesia, Malaysia, Myanmar, Philippines, Thailand and Vietnam (Demski et al. 1993).
Biology and transmission
PStV particles are filamentous flexuous rods, approx. 752nm long and 12 nm in diameter. Each particle consists of single protein pieces of 33500 daltons. The genome is single stranded (ss) positive-sense RNA molecule of about 9500 nucleotides. The particles are relatively stable and can be stained with 2% phosphotungstate or ammonium molybdate pH 6.5 (Demski et al. 1993). The virus is transmitted by several species of aphids in a non-persistent manner, which is also the only means of disease spread under field conditions. Aphis craccivora is the major vector for the transmission of PStV. Apart from A. craccivora, Myzus persicae and A. gossypii, and Hysteroneura setariae have been shown to be highly efficient PStV vectors for the transmission of the disease. PStV transmission through groundnut seed can be as high as 37% in artificially inoculated plants (Demski et al. 2004). Under natural conditions, however, the transmission frequency is up to 7%. PStV seed transmission frequency can be influenced by the virus isolate, groundnut cultivar and environment. The virus can be detected in both the embryo and the cotyledon, but not in the seed testa.
Detection/indexing methods at ICRISAT
- Pre export field inspection and ELISA (Sudarshan and Reddy 1989) are used.
Treatment/control
- Not known.
Procedures followed in case of positive test at ICRISAT
- Rejection of seed samples in case of positive test.
References and further reading
Adalla CB, Natural MP. 1988. Peanut stripe virus disease in the Philippines. In: ICRISAT, ed. Coordination of Research on Peanut Stripe Virus: Summary Proceedings of the First Meeting to Coordinate Research on Peanut Stripe Virus Disease of Groundnut, 9-12 June 1987, Malang, Indonesia. Patancheru, Andhra Pradesh, India: ICRISAT, pp9.
Demski JW, Reddy DVR, Sowell Jr. G, Bays D. 2004. Peanut stripe virus - a new seed-borne potyvirus from China infecting groundnut (Arachis hypogaea) . Annals of Applied Biology 105: 495-501.
Demski JW, Reddy DVR, Wongkaew S, Xu Z, Kuhn CW, Cassidy BG, Shukla DD, Saleh N, Middleton KJ, Sreenivasulu P, Prasada Rao RDVJ, Senboku T, Dollet M, McDonald D. 1993. Peanut stripe virus. Information Bulletin No. 38. Patancheru, Andhra Pradesh, 502 324, India: International Crops Research Institute for the Semi-Arid Tropics, Griffin, GA 30223, USA: Peanut Collaborative Research Support Program, 20pp.
Middleton KJ, Saleh N. 1988. Peanut stripe virus disease in Indonesia and the ACIAR Project. In: ICRISAT, ed. Coordination of Research on Peanut Stripe Virus: Summary Proceedings of the First Meeting to Coordinate Research on Peanut Stripe Virus Disease of Groundnut, 9-12 June 1987, Malang, Indonesia. Patancheru, Andhra Pradesh, India: ICRISAT, 4-6.
Sudarshana MR, Reddy DVR. 1989. Penicillinase-based enzyme-linked immunosorbent assay for the detection of plant viruses. Journal of Virological Methods 26: 45-52.
Varma A, Jain RK, Ghewande MP, Nand Gopal V. 1994. Virus dieases of groundnut in India with particular reference to peanut stripe virus. In: Reddy DVR, McDonald D, Moss JP, eds. Working Together on Groundnut Virus Diseases: Summary and Recommendations of International Working Groups on Groundnut Virus Diseases, 15-19 August 1993, Scottish Crop Research Institute, Dundee, UK. Patancheru, Andhra Pradesh, India: ICRISAT, 61-62.
Wongkaew S, Dollet M. 1990. Comparison of peanut stripe virus isolates using symptomatology on particular hosts and serology. Oleagineux (Paris) 45: 267-278.
Xu Z, Zhang Z, Chen K, Reddy DVR, Middleton KJ, Chen J, Wightman JA. 1994. Current research on groundnut virus diseases in China. In: Reddy DVR, McDonald D, Moss JP, eds. Working Together on Groundnut Virus Diseases: Summary and Recommendations of a Meeting of International Working Groups on Groundnut Virus Diseases, 15-19 August 1993, Scottish Crop Research Institute, Dundee, UK; Patancheru, Andhra Pradesh, India: ICRISAT, 59-60.
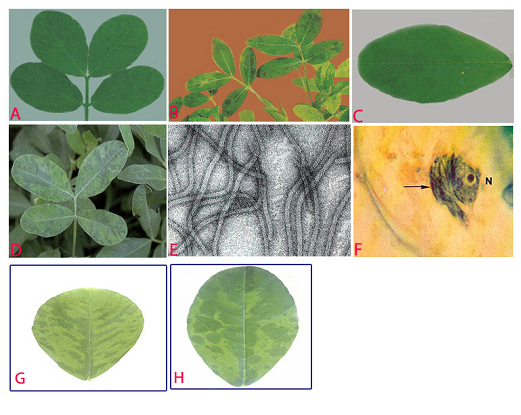
Peanut stripe (Peanut stripe virus) of groundnut: (A)stripe and green banding symptoms; (B)striping and mosaic (oak leaf pattern) symptoms in older leaves; (C)chlorotic flecks; (D)chlorotic ring mottle symptoms; (E)electron micrograph (x 257000); (F)subdivision-IV type inclusive body (arrow) adjacent to the nucleus (N) in PStV infected leaflet; (G)necrotic strips and (H)mild mottle and blotch (photos:ICRISAT) |
Scientific name
Peanut stunt virus(PSV).
Other scientific names
Robinia mosaic virus, Black locust true mosaic virus, Clover blotch virus
Importance
High
Significance
In the 1960s, PSV was a problem in Virginia, North Carolina and Georgia, but it is not of economic importance for groundnut production in the USA. However, PSV sporadically causes a high incidence of peanut stunt disease in Hebei, Henan and Liaoning provinces in China (Xu et al.1992).
Symptoms
Symptomsvary depending on the host plant and the strain of the virus. In groundnuts, there are various degrees of stunting, shortening of the petioles, reduced leaf size, mild mottling and malformation of pods. Seeds from infected plants appear deformed, frequently with a split pericarp wall, and have poor viability.
Hosts
PSV naturally infects several leguminous host species - Arachis hypogaea (groundnut), Vicia faba (broad bean), Glycine max (soybean), Pisum sativum (pea), Vigna unguiculata (cowpea), Lupinus luteus (yellow lupin), Nicotiana tabacum (tobacco), Lycopersicon esculentum (tomato) and Datura stramonium (devils trumpet).
Geographic distribution
PSV is world wide in distribution, including several countries in Europe (France, Hungary, Italy, Poland and Spain; in Asia (China, Georgia, Japan, Korea and India); in Africa (Morocco and Sudan) and also in USA (Subrahmanyam et al. 1992).
Biology and transmission
PSV particles are isometric or polyhedral, with a diameter of ca 25-30 nm. The coat protein of PSV contains a single polypeptide with an apparent molecular weight of about 26 kDa. PSV has a positive-sense tripartite genome (designated RNAs 1, 2 and 3 in order of decreasing size). In addition to the genomic RNAs, the virions also encapsulate a fourth RNA (called RNA 4) which is a subgenomic RNA that functions as mRNA for the viral-coat protein (Naidu et al. 1995 ). Three types of native particle exist, each consisting of the same protein shell, yet containing different RNA species. One type of particle contains genomic RNA 1, another contains RNA 2 and the third contains genomic RNA 3 and subgenomic RNA 4. However, all the particles have the same sedimentation coefficient (S20w). All three genomic RNAs, but not subgenomic RNA 4, are essential for infection. Certain naturally occurring PSV isolates also encapsidate a satellite RNA (satRNA) molecule with its genomic and subgenomic RNAs (Naidu et al. 1995). PSV-associated satRNAs are linear, single-stranded RNA molecules, ranging in size from 391 to 393 nucleotides. PSV satRNA has essentially no sequence homology with its helper virus (i.e. PSV) genomic RNAs (Collmer et al. 1985). Depending on the PSV strain and host species involved, satRNAs can modulate the symptoms caused by PSV (Naidu et al. 1992). PSV supports the replication of its satRNAs but not those associated with cucumber mosaic virus. PSV is transmitted in nature by insect vectors - Aphis craccivora, A. spiraecola and Myzus persicae. It is transmitted in the non-persistent manner. PSV can also be transmitted by mechanical inoculation. PSV is transmitted in a small percentage (0.1%) of groundnut seeds (Kuhn 1969).
Detection/indexing methods at ICRISAT
- Pre export field inspection and double-antibody-sandwich (DAS)-ELISA and an indirect ELISA are used to detect PSV (Bharathan et al. 1984).
Treatment/control
- Not known
Procedures followed case of positive test at ICRISAT
- Incineration of the infected plants and rejection of the infected seeds are applied.
References and further reading
Bharathan N, Reddy DVR, Rajeshwari R, Murthy VK, Rao VR, Lister RM. 1984. Screening peanut germplasm lines by enzyme-linked immunosorbent assay for seed transmission of peanut mottle virus. Plant Disease 68: 757-758.
Collmer CW, Hadidi A, Kaper JM. 1985. Nucleotide sequence of the satellite of peanut stunt virus reveals structural homologies with viroids and certain nuclear and mitochondrial introns. Proceedings of the National Academy of Sciences USA 82: 3110-3114.
Kuhn CW. 1969. Effects of peanut stunt virus alone and in combination with peanut mottle virus on peanut. Phytopathology 59:1513-1516.
Naidu RA, Collins GB, Ghabrial SA. 1992. Peanut stunt virus satellite RNA: analysis of sequences that affect symptom attenuation in tobacco. Virology 189: 668-677.
Naidu RA, Hu CC, Pennington RE, Ghabrial SA. 1995. Differentiation of eastern and western strains of peanut stunt cucumovirus based on satellite RNA support and nucleotide sequence homology. Phytopathology 85: 502-507.
Subrahmanyam P, Wongkaew S, Reddy DVR, Demski JW, McDonald D, Sharma SB, Smith DH. 1992. Field diagnosis of groundnut diseases. Information bulletin no. 36, Patancheru, AP, 502 324, India: International Crops Research Institute for the Semi Arid Tropics. 84pp.
Xu Z, Zhang Z, Chen K, Chen J. 1992. Characteristics of strains of peanut stunt virus by host reactions and pathogenicity to peanut. Oil Crops of China 4: 25-29.
 Peanut stunt (Peanut stunt virus) of groundnut: dwarfing symptoms of branches (photo:ICRISAT) |
Scientific name
Peanut clump virus(PCV).
Other scientific name
Indian Peanut Clump Virus (IPCV)
Importance
High
Significance
PCV infected plants do not produce pods, and yield losses in groundnut grown in light sandy soils are as high as 60 % even in late infected crops (Reddy et al. 1988).
Symptoms
Plants affected by clump disease are conspicuous in the field because of their severe stunting and dark green appearance. Initial symptoms appear on young leaflet as mottling, mosaic and chlorotic rings, but later turn dark green with or without faint mottling as the leaves mature. Early infected plants become severely stunted. Late infected plants may not show concipous stunting but appear dark green with faint mottling on younger leaflets. In late infected plants, clumping may be restricted to few branches. Infected plants become bushy and produce several flowers. Early infected plants may not produce any pods and late infected plants may produce poorly developed pods (Reddy et al. 1988). These plants often occur in patches and the disease reoccurs in the same area of the groundnut field in successive years.
Hosts
PVC causes disease in Triticum (wheat), Hordeum vulgars (barley), Saccharum officinarum (sugarcane), Capsicum spp (chilli), and Cajanus cajan (pigeonpea). It also infects Sorghum bicolor (sorghum), Zea mays (maize), Oryza sativa (rice), Brassica juncea(mustard), Glycine max (soybean) and Vigna radiate (mung bean), but these hosts do not exhibit symptoms (Thouvenel and Fauquet 1981).
Geographic distribution
The disease affects groundnut in several countries in western Africa including Burkina Faso, Ivory Coast and Senegal, and in several countries of Asia, including India and Pakistan, and also in South Africa (Delfosse et al. 1995).
Biology and transmission
IPCV particle dimensions in leaf dip preparations are 184±8 ´ 24±2 nm in uranyl acetate and 169 ± 5 and 239 ± 13 ´ 20±1 nm in phosphotungstate (Thouvenel and Fauquet 1981). In IPCV-Ludhiana strain had 175 nm particles contains RNA-2 (1.35 ´ 106 mol. wt) and 235 nm particles contain RNA-1(1.84 ´ 106mol. wt) since both size of particles are needed to induce local lesions in bean tissues. IPCV isolates from India have been grouped into 3 distinct serotypes - IPCV-H (Hyderabad), IPCV-D (Durgapura) and IPCV-L (Ludhiana). Complementary DNA hybridization tests have shown that isolates D, H and L of IPCV are related to each other but not to furoviruses (Robinson and Reddy 1985). IPCV is seed transmitted up to 11% in groundnut and also through seeds of finger millet, pearl millet, fox tail millet, wheat and maize. IPCV has been transmitted by Polymyxa graminis (Ratna et al. 1991).
Detection/indexing methods at ICRISAT
- Pre export field inspection, and Double antibody sandwich ELISA (DAS-ELISA) and Nucleic acid hybridization (Reddy et al. 1985) tests are used.
Treatment/control
- Seed treatment is not available but satisfactorily controlled by adopting proper cultural practices over a period of time.
Procedures followed in case of positive test at ICRISAT
- Incineration of the infected crop and rejection of the infected seeds are used in case of positive test.
References and further reading
Delfosse P, Bashir M, Malik SN, Reddy AS. 1995. Survey of groundnut viruses in Pakistan. International Arachis Newsletter 15: 51-52.
Ratna AS, Rao AS, Reddy AS, Nolt BL, Reddy DVR, Vijaylakshmi M, McDonald D. 1991. Studies on transmission of peanut clump virus disease by Polymyxa graminis. Annals of Applied Biology 118: 71-78.
Reddy DVR, Robinson DJ, Roberts IM, Harrison BD. 1985 Genome Properties and Relationships of Indian Peanut Clump Virus. Journal of General Virology 66: 2011-2016
Reddy DVR, Nolt BL, Hobbs HA, Reddy AS, Rajeswari R, Rao AS, Reddy DDR, McDonold D. 1988. Clump virus in India: Isolates, host range, transmission and management. In: Viruses with fungal vectors.(Cooper JI, and Asherr, MJCeds) Asso. Appl. Siol. Wellesbourne , UK, pp. 239-246.
Robinson DJ, Reddy DVR.1985. Nucleic acid hybridization tests for relationships among furovirus isolates. In: 5th Ann. Rep., Scottish Crops Research Institute, Invergowrie, Dundee, Scotland, 155.
Thouvenel JC, Fauquet C. 1981. Further properties of peanut clump virus and studies on its natural transmission. Annals of Applied Biology 97: 99-107.
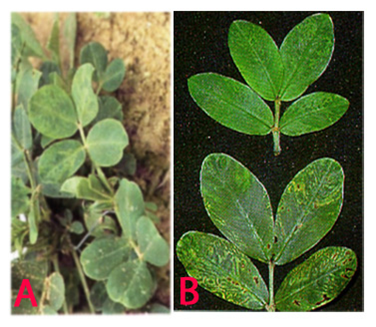 Peanut clump (Peanut clump virus) of groundnut: (A)mosaic and mottling symptoms; (B)darker green and faint mottling older leaves and chlorotic rings (photos:ICRISAT) |



 stog-groundnut
stog-groundnut
