Forage legume
Health diagnosis of forage legume genetic resources
Contributors to this page: ILRI, Ethiopia (Jean Hanson, Juvy B. Cantrell, Janice Proud); CIAT, Cali, Colombia (Maritza Cuervo); ICARDA, Syria (Siham Asaad, Ahmed Amri, Kenneth Street, Ali Shehadeh, Natalya Rukhkyan); Bioversity International/ILRI, Addis Ababa, Ethiopia (Alexandra Jorge).
|
A field inspection of grasspea (photo: ICARDA)
Visual virus inspection (photo: ILRI) |
List of pests and diseases of quarantine importance
Forage legumes are susceptible to a wide range of pests and diseases. Click here for more detailed and updated information from the page on the safe movement of germplasm, on this website.
Click the forage legume health table for specific species information about health diagnosis methods to detect some of these diseases.
Options for testing procedures
Recommended methods to detect the presence of each pest or disease:
- Virus (ELISA, TBIA).
- Ascochyta spp. (freezing blotter method, PDA).
- Phoma spp. (freezing blotter method, PDA).
- Botrytis cinerea (PDA).
- Colletotrichum sp. (blotter method).
- Fusarium oxysporum (blotter method).
- Rhizoctonia solanum (blotter method).
- Penicillium sp. (blotter method).
- Ditylenchus dipsaci (nematode test).
- Orobanche spp. (filter wash test).
- Cuscuta spp. (filter wash test).
Testing intervals/seasons
|
|
Testing before material goes into the genebank or to the field is important to reduce transfer of diseases or pests.
Virus
- Test seedlings before transfer to the field for regeneration or during regeneration and rogue infected material.
Fungus
Ascochyta spp., Phoma spp., Botrytis cinerea.
- Test seed lots on entry to genebank.
- Perform field inspection at all stages and rogue infected plants or/and seed.
Nematodes
Ditylenchus dipsaci.
- Perform field inspection at all stages and rogue infected plants or/and seed.
Weeds
Orobanche spp. and Cuscuta spp.
- Perform field inspection at flowering stage, rogue before seed dispersal and burn the plants.
Recording information during health diagnosis
|
The text for this flip book was extracted from: Rao NK, Hanson J, Dulloo ME, Ghosh K, Nowel D, Larinde M. 2006. Manual of seed handling in genebanks. Handbooks for Genebanks No. 8. Bioversity International, Rome, Italy. 147pp. |
The following information should be recorded for each health diagnosis step:
- Accession number (ID number).
- Lot number (ID number).
- Taxonomic identification.
- Date of test (the date that the test was commenced).
- Number of replications (the number of replicates in the test).
- Number of seeds/plants per replication (the number of seeds/plants in each replicate of the test).
- Pre-treatments (pre treatments used for the test).
- Media [the media for the test (fungi)].
- Pathogen tested (name of pathogen tested).
- Test method (method used).
- ID in health diagnosis experiment, e.g. Plot numbers (plot ID number) or entry number of experiment.
- Percentage infection (% of seeds or plants infected).
References and further reading
Albrechtsen SE. 2006. Testing Methods for Seed-Transmitted Viruses: Principles and Protocols. CABI Publishing, Cambridge, USA. pp. 268.
Nan ZB, Hanson J. 1998. Detection of seed borne fungi in Stylosanthes hamata, S. guianensis and S. scabra. Seed Science and Technology 26:333-345.
Nan ZB, Hanson J, Yeshi MW. 1998. Effects of sulphuric acid and hot water treatments on seed borne fungi and germination of Stylosanthes hamata, S. guianensis and S. scabra. Seed Science and Technology 26:33-43.
Rao NK, Hanson J, Dulloo ME, Ghosh K, Nowel D, Larinde M. 2006. Manual of seed handling in genebanks. Handbooks for Genebanks No. 8. Bioversity International, Rome, Italy. Available in English (1.5 MB), Spanish (1.4 MB) and French (1.9 MB).
Distribution of forage legume genetic resources
Contributors to this page: ILRI, Ethiopia (Jean Hanson); ICARDA, Syria (Ahmed Amri, Kenneth Street, Ali Shehadeh, Natalya Rukhkyan); GRCTPL, Australia (Richard Snowball); Bioversity International/ILRI, Addis Ababa, Ethiopia (Alexandra Jorge).
|
Contents: |
Policies and regulations for distribution
Common policies on distribution and access to plant material
- Follow the International Treaty on Plant Genetic Resources for Food and Agriculture (ITPGRFA) for in-trust germplasm using the SMTA (SMTA: ITPGRFA, SGRP) and Plant Breeders' Rights (click for more information from Australia, Canada and the UK) for varieties (CGIAR centres have signed an agreement with the ITPGRFA).
Policy exceptions
- Repatriation of germplasm is done whenever material is available (material should always be repatriated as a special case).
National laws and regulations
It is essential to follow the terms and conditions in the host country agreements:
- Export permits.
- Phytosanitary certificate.
- Certificate of origin.
International laws and regulations
- The seed shipment should be sent with the Standard Material Transfer Agreement (SMTA: ITPGRFA, SGRP) even for non Annex I species using appropriate footnotes (CGIAR Centres have signed an agreement with the ITPGRFA which covers use of the SMTA).
Phytosanitary regulations
- Phytosanitary certificates are needed for most countries.
- See the STOGs section in this website for seed health movement (this is essential to avoid the spread of pests and diseases).
User related issues for distribution
Feedback to users
Describes factors that can influence the delivery of the plant material:
- Respond to requests with lists of material, forms and conditions of access, SMTA (ITPGRFA, SGRP) as soon as possible after receipt of the request (users may not know about the conditions so it is better to inform them before proceeding with the request).
- Provide passport and germination data with requests.
Describes recommended procedures that ensure the material distributed matches the client request:
- On specific requests, match accession numbers with specified request.
-
If accession numbers are not specified, match accessions to users' needs. These could include:
- Species.
- Plant habit to fit the crop system.
- Environment.
- Use (grazing, cut and carry, soil stabilization).
Feedback from users
Describes the most relevant information required to be received from users:
- Information on characterization and evaluation/use (information on performance in one area allows better selection of germplasm for similar areas).
Quantity of material recommended to be distributed
- It must be sufficient to cover the diversity in the accession and produce material for future use (users should have access to the full diversity within the accession and sufficient seeds to multiply to obtain a genetically similar sample for future use. The provision of small quantities maintains the stocks and reduces the regeneration frequency).
- Cultivated species: 100 seeds.
- Wild species: 50 seeds.
|
|
Checking availability
Availability in stock
- Check availability of seeds in stock and verify their availability.
- Distribution of the requested seeds should not cause the accession to fall below the minimum stock.
- Accessions with low amounts of seeds in stock should not be distributed.
Checking passport data
- Passport data can be checked to ensure the species is adapted to the requestor’s needs (this avoids waste of seeds by sending material that the requestor does not need or want).
Preparing accessions for distribution
Registering the request
- Give consecutive numbers in order to track requests (this allows requests to be handled on a first come first served basis).
Preparing lists of accessions available
- Generate lists of accessions that meet users' needs.
Checking requirements for Material Transfer Agreements (MTAs)
- All Annex I and non-Annex I in-trust material must be sent with the SMTA (ITPGRFA, SGRP).
- Otherwise use an appropriate MTA.
Generating labels for accessions
- Print labels with the most important information: accession number, taxonomic identification, origin.
- Use the database and print labels to avoid errors.
Labelling the accession containers
- Use labels with good adhesive and clear printing (this avoids errors and mixing during shipping).
Removing containers from the genebank and acclimatization procedures required
- Allow all seeds to warm to room temperature before opening containers and seal seeds again as quickly as possible.
- Packets should be sealed as quickly as possible to avoid uptake of moisture (condensation will form on cold seeds and cause changes in moisture content).
Assuring accuracy in identification
- Staff should double check all labels and seeds against lists for accuracy and to avoid errors.
- The use of barcoding technology reduces errors.

Check packets against lists to avoid errors (photo: ILRI)
Extracting samples from the original containers
- Use a clean spatula, take care not to mix samples and do not leave containers open for long periods (cleanliness and care are needed to avoid errors through mixing).
- Packets should be sealed and containers closed as quickly as possible to avoid uptake of moisture.
Preparing the information list to accompany the plant material
The following basic information is important for the user
-
Passport data:
- Accession number.
- Accession identification.
- Crop name.
- Taxonomic identification.
- Country of origin.
- Biological status.
- Collecting location.
- Source.
- Germination data showing viability and testing method.
- Characterization data used to verify accessions should be provided upon request.
Cover letter:
- Remind users of the terms and conditions of access and request feedback (it is important to make contact with the user for future feedback).
Dispatching the plant material
Packaging
- Pack the seeds in paper envelopes or laminated aluminium foil envelopes or nylon bags (plastic bags are suitable for short periods to avoid packets bursting and mixing seeds or getting wet during transit).
- Then pack seeds and attached documents e.g. the seed list, SMTA, phytosanitary certificate, import permit, GMO-free certificate in a plastic bag and then in a strong envelope or a cardboard box.
- Attach a copy of the phytosanitary certificate, import permit and list of materials to the outside of the box if material is being dispatched to another country.
- A copy of the SMTA must be attached to the outside of the envelope or box. Use of the material constitutes agreement with the terms of the SMTA.
- Label the envelope/box with the complete mailing address of the requester.

Packing seeds for distribution (photo: ILRI)
Reply form
- Include a reply form in the shipment (a reply form should be returned by the requester to acknowledge that the seeds have been received and in good condition).
Sending the plant material
- Use courier or other rapid means of transit (the method of transport should avoid heating and delays in transport).
Recording shipping details
- Record date and method of shipping (shipping details are important for tracking during shipping).
Updating the genebank inventory
- Deduct the weight of the seeds sent from the remaining stock (needed to update stock control).
The following information must be recorded for each consignment:
- Reference number.
- Crop name.
- Consignee’s name and designation.
- Name and address of organization.
- User information (type of organization requesting material).
- Date of request.
- Date of supply.
- Accession number and quantity of samples provided.
- Phytosanitary certificate.
- Export permit number.
- Reference number of SMTA.
- Classification of intended germplasm use.
System for tracking material/inventory system for distribution
- Update related data tables in the database management system (a database system allows easy and fast access to data and allows macros to be written for routine operations).
- Use labels with good adhesive and clear printing (this avoids errors and mixing during shipping).
References and further reading
Agreement sample form. Download document.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD, editors. 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Available here.
List of crops covered under the multilateral system Annex I.
Standard Material Transfer Agreement (ITPGRFA, SGRP)
The International Treaty on Plant Genetic Resources for Food and Agriculture [homepage of theITPGRFA] [online]. Available from: http://www.planttreaty.org/.
Viability of forage legume genetic resources
Contributors to this page: ILRI, Ethiopia (Jean Hanson); ICARDA, Syria (Ahmed Amri, Kenneth Street, Ali Shehadeh, Natalya Rukhkyan); GRCTPL, Australia (Richard Snowball); Bioversity International/ILRI, Addis Ababa, Ethiopia (Alexandra Jorge); CIAT, Cali, Colombia (Daniel Debouck).
|
Contents: |
Laboratory method
 Seed germination tests (photo: ILRI) |
Viability test methods are specific for different species. International Seed Testing Association rules should be used when available. Specific information is available for many species (see Species Compendium).
Type of test
- Standard germination tests (see list for species specific information).
or
- Sequential germination tests (these are less accurate, but require less seeds and are suitable in case of limited number of seeds). Use at least 40 seeds per test. If less than 30 seeds germinate, programme the accession for regeneration.
Number of seeds and replicates
|
The text for these flipbooks was extracted from: Rao NK, Hanson J, Dulloo ME, Ghosh K, Nowel D and Larinde M. 2006. Manual of seed handling in genebanks. Handbooks for Genebanks No. 8. Bioversity International, Rome, Italy. 147pp.
|
International Seed Testing Association rules should be used when available.
- ISTA recommends four replications of 100 seeds.
- A minimum number of four replications of 50 seeds is acceptable, when seeds are limited.
- The number of seeds should be practical (depending on the seeds available) and enough to provide statistically significant results.
- Wild species often have limited numbers of seeds to use for testing.
- For the sequential test use 40 seeds.
Pre-treatment
International Seed Testing Association rules should be used when available.
- Viability test methods are specific for different species (see list for specific information).
Media
International Seed Testing Association rules should be used when available.
- Viability test methods are specific for different species (see list for specific information).
Temperature
International Seed Testing Association rules should be used when available.
- Viability test methods are specific for different species (see list for specific information).
Light
International Seed Testing Association rules should be used when available.
- Viability test methods are specific for different species.
- A 12 hour light period should be used to simulate tropical day length conditions.
Duration of test
International Seed Testing Association rules should be used when available.
- Viability test methods are specific for different species (see list for specific information).
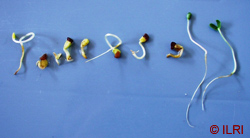
The picture above shows abnormal (left) and normal (the last two on the right) legume seedlings. (photo: ILRI) |
Others
- Germination is complete when the seedling can be judged as normal (ISTA, 2006), i.e. having a developed shoot and root (see image below).
Monitoring intervals
Legume seeds are usually long-lived. The sampling interval should be spaced so as not to allow seeds to fall below 65% viability but not so frequent as to waste seeds for unnecessary testing:
- Every ten years above 80% germination.
- Every five years below 80% germination or when longevity is not known.
- Seeds should be regenerated when viability reaches 65%.
Recording information during viability testing
The following information must be recorded for each testing step:
- Accession number (ID number).
- Lot number (ID number).
- Taxonomic identification.
- Date of test (the date that the test was commenced).
- Number of replications (the number of replicates in the test).
- Number of seeds per replication (the number of seeds in each replicate of the test).
- Pre-treatment (pre-treatments used for the test. More than one can be used in conjunction).
- Media (the media for the test).
- Temperature (the temperature used during the test in degrees centigrade. If an alternate temperature is used this temperature usually refers to the temperature during the day period, unless stated otherwise).
- Alternate temperature (if more than one temperature is used, the temperature in degrees centigrade of the alternate period. The alternate temperature usually corresponds to the dark period).
- Length of dark (the number of hours that there is no light. This usually corresponds to the period at lower temperature, when two temperatures are used).
- Length of test (the number of days during which the test was continued).
- Percentage germination (the average percentage germination over all replicates, calculated from the number germinated per replicate).
- Tolerance (a measure of the statistical accuracy of the test according to the standard tolerance tables).
- Percentage dormancy (the average percentage of hard seeds which are not rotten, over all replicates).
- Test reference (a consecutive number given each time that seed lot is tested over time).
Describes the recommended monitoring methods to assure minimum viability and quantity of seeds in storage.
- Do routine tests for seed viability at regular intervals (monitoring methods for viability should minimize use of seeds while providing a good indication of reduction of viability).
- The stock control system should be queried to determine accessions for regeneration.
-
Accessions can be flagged (marking accessions in the database helps to avoid distribution of accessions by mistake and quickly identify accessions for regeneration) in the database as:
- (A) Accession is available for distribution.
- (L) Low stock; the accession is available for distribution but regeneration should be planned.
- (S) Low number of seeds; no distribution is allowed; the sample should be regenerated.
- Genebank facilities should be checked for good working order or damage and preventive maintenance should be done on equipment (regular monitoring of equipment and buildings allows early identification of problems and preventive maintenance before failure).
Monitoring frequency
- This is set according to the minimum quantity and minimum viability of seeds below which they need to be regenerated.
Critical quantity
- A minimum of 600 seeds (this quantity allows two regeneration attempts using 300 seeds giving a high probability to have 100 plants to maintain genetic integrity of accessions, even for seeds with 50% viability).
- Where possible it is better to regenerate when seed quantities reach 1000 to mitigate risks.
Critical germination level
- 65% germination percentage for wild species (recommended by FAO/IPGRI, 1994).
Recording information during routine monitoring
The following information should be recorded for each step:
- Accession number (ID number).
- Lot number (ID number).
- Weight of seeds (weight of seeds on hand).
- 1000 seed weight (weight of one thousand seeds to be used for calculating number of seeds from weights).
- Percentage of germination (the average percentage germination over all replicates, calculated from the number germinated per replicate).
- Stock control flag (code to indicate the stock level and distribution).
- Availablity of material flagged (code to indicate the stock level – see above)
- Regeneration flag (code to indicate if regeneration required).
References and further reading
Akinola JO, Afolayan RA, Olorunju SAS. 1991. Effects of storage, testa colour and scarification method on seed germination of Desmodium velutinum (Willd.) DC. Seed Science and Technology 19:159-166.
FAO/IPGRI. 1994. Genebank standards. Food and Agriculture Organization of the United Nations, Rome and International Plant Genetic Resources Institute, Rome. Available in English, Spanish, French and Arabic.
International Seed Testing Association [homepage of (ISTA)] [online]. Available from: http://www.seedtest.org/en/home.html. Date accessed: 17 Jan 2011.
ISTA. 2006. Handbook on Seedling Evaluation, 3rd Edition.
Souza FHD, Marcos-Filho J. 1993. Physiological characteristics associated with water imbibition by Calopogonium mucunoides Desv. seeds. Seed Science and Technology 21:561-572.
Thormann I, Metz T, Engels JMM. 2004. The Species Compendium (release 1.0; December 2004). [online] Available from: http://www.bioversityinternational.org/databases/species_compendium_database/index.html. Date accessed: 09 April 2013.
Wang YR, Hanson J. 2008. An improved method for breaking dormancy in seeds of Sesbania seban. Cambridge University Press. Experimental Agriculture. Vol. 44, pp. 185–195. Abstract available from: http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=1842236. Date accessed: 17 Jan 2011.
Wang YR, Hanson J. 2008. The impact of temperature on seed germination in diverse accessions of 4 wild Vigna species. Proceedings of the XXI International Grasslands Congress, Multifunctional Grasslands in a Changing World. Hohhot, China, June 2008, p. 557.
Wang YR, Hanson J, Wolde YM. 2007. Effect of sulfuric acid pretreatment on breaking hard seed dormancy in diverse accessions of five wild Vigna species. Seed Science and Technology 35:550-559.
Storage of forage legume genetic resources in seed banks
Contributors to this page: ILRI, Ethiopia (Jean Hanson); ICARDA, Syria (Ahmed Amri, Kenneth Street, Ali Shehadeh, Natalya Rukhkyan); GRCTPL, Australia (Richard Snowball); Bioversity International/ILRI, Addis Ababa, Ethiopia (Alexandra Jorge).
|
Contents: |
When it should be used
- All original samples should be placed in long-term storage (original samples are the best representative sample of that accession and storage conditions should be used to retain viability as long as possible).
- In addition, representative samples of all accessions should be stored in base collections for long-term storage.
Sample specifications
Minimum sample size/viability for storage
- 4000 seeds or 5 g for small seeds of 90% germination or 80% germination for wild species (see FAO/IPGRI, 1994).
Moisture content:
- 3-7% (according to FAO/IPGRI, 1994).
Container specifications
Seed packaging method
- Laminated re-sealable aluminium foil packets are moisture proof and very practical, making good use of space.
- Use of vacuum for packing is optional.
- Packing is best carried out in an air-conditioned room with controlled humidity as soon as possible after drying (rapid packing in a dry environment prevents reabsorption of moisture by the seeds during packing).
Specifications of packaging material
Minimum quality: outer polyester of 12 µm thickness, middle aluminium layer of 9 µm thickness and inner polythene layer of 55 µm (this thickness is impermeable and sufficiently flexible for handling and strong enough for forage legume seeds, which are usually smooth).
Storage specifications
The two commonly available options for seed storage are walk-in cold stores and freezers. The choice depends on the number of accessions to be stored, seed size and storage temperatures selected.
- When collections are small and sub-zero temperatures are required, chest or upright freezers are a cheaper option for seed storage.
Assigning location codes
- Location codes should specify the exact place in the store (e.g. unit/block number, row number, shelf number and tray/box number) or freezer (e.g. freezer shelf and box number). The exact locations are very important to locate material in the store.
- Moveable shelves on tracks are preferred (moveable shelves make best use of space).
Storage conditions
- At -18oC to -20oC (FAO/IPGRI, 1994).
Active collection
|
|
When it should be used
To store seeds for distribution and use (seed quantities for distribution are usually larger and are not usually stored for long periods, therefore storage conditions are less stringent).
Sample specifications
Minimum sample size/viability for storage
- A minimum of 1500 seeds and up to 500 g and above 80% of original germination (sufficient seeds for distribution needs during the period of high viability).
Moisture content
- 3-7% (according to FAO/IPGRI, 1994).
Container specifications
Seed packaging method
- Laminated re-sealable aluminium foil packets are moisture proof and very practical, making good use of space.
- Plastic or metal containers if store is dehumidified (store must be dehumidified to avoid absorption of moisture from the air surrounding the seeds).
Specifications of packaging material
- Laminated aluminium foil: Minimum quality: outer polyester of 12 µm thickness, middle aluminium layer of 9 µm thickness and inner polythene layer of 55 µm (this thickness is impermeable, sufficiently flexible for handling and strong enough for forage legume seeds, which are usually smooth).
- Plastic or metal containers: Square-based bottles with a screw cap and inner rubber seal made of pure plastic materials (high quality), semi transparent, white in colour, adequate volume or metal cans of similar design.
Storage specifications
Assigning location codes
- Location codes should specify the exact location in the store, e.g. unit/block number, row number, shelf number and tray/box number (exact locations are important to locate and access material in the store).
- Moveable shelves on tracks are preferred (moveable shelves make best use of space).
Storage conditions
- Between 0 to 10oC (conditions to maintain seed viability for at least 25 years).
For information on safety duplication, click here.
Storage management
Storage space arrangement
- Moveable racks (this maximizes space between and on the racks in the store and is the most economic way to utilize the space).
- Shelves should be spaced slightly more than the height of the containers.
System for tracking material/inventory system
- Database of stock and location and use of barcodes (databases are needed to keep track of information. Barcodes help avoid errors in recording).
Recording information during conservation
The following information must be recorded for each accession:
- Accession number (ID number).
- Lot number (ID number).
- Weight of seeds (weight of seeds in store).
- Number of seeds (number of seeds in store).
- Thousand or hundred seed weight (weight of 1000 or 100 seeds).
- Number of packets (number of containers for seeds of one lot of one accession).
- Location of containers (store, shelf or box number).
- Type of container (material and size of container).
- Year of production of seeds (indicates age of seeds).
- Flag for regeneration [Y/N].
- Flag indicating seed availability and distribution status [A, L, S].
- Year of safety duplication (year).
- Institute holding the duplicate (name of institute holding the safety duplicate).
- Location of duplicate sample (box label where the duplicate sample is placed).
References and further reading
Bass LN. 1984. Storage of seeds of tropical legumes. Seed Science and Technology 12:395-402.
Cabrales R, Bernal J. 1983. Effect of different systems of seed treatment, packing, and storage on vigor and germination of five tropical forage legumes. XIVth International Grasslands Congress. pp. 263-265.
Chin HF, Hanson J. 1999. Seed storage. In: Loch DS, Ferguson J, editors. Forage Seed Production Vol II Tropical and Subtropical Species. CABI Publishing, UK. pp. 303-315.
FAO/IPGRI. 1994. Genebank standards. Food and Agriculture Organization of the United Nations, Rome and International Plant Genetic Resources Institute, Rome. Available in English, Spanish, French and Arabic.
Keya NCO, van Eijnatten CLM. 1975. Studies on oversowing of natural grasslands in Kenya 1. The effects of seed threshing, scarification and storage on the germination of Desmodium uncinatum (Jacq.) DC. East African Agriculture and Forestry Journal 40:261-263.
Penteado MI de O, Sáenz de Mira LE, Pérez de la Vega M. 1996. Genetic resources of Centrosema spp.: genetic changes associated to the handling of an active collection. Genetic Resources and Crop Evolution 43: 85 90.
Sample processing of forage legume genetic resources
Contributors to this page: ILRI, Ethiopia (Jean Hanson); ICARDA, Syria (Ahmed Amri, Kenneth Street, Ali Shehadeh, Natalya Rukhkyan); GRCTPL, Australia (Richard Snowball); Bioversity International/ILRI, Addis Ababa, Ethiopia (Alexandra Jorge).
|
Contents: |
This is the removal of physical contaminants from plant materials after harvesting, before storage. Legume seeds are hard and there is no physical damage during cleaning. All contaminants should be removed because of space constraints and costs of storage.
Legume pods should be threshed and cleaned immediately after harvest or soon after they arrive at the genebank to avoid infestation with bruchid insects. Many legume pods are dehiscent and open readily during drying when spread out in thin layer with sufficient air circulation.
|
Drying pods of forage legumes before threshing (photo: ILRI) |
- Seeds of Stylosanthes and Desmodium are firmly attached to pods and require threshing to extract the seeds.
- Threshing should be done when seed moisture content is between 12% and 16% to minimize injury to the seeds.
- Pods can be air dried by spreading in a thin layer in the shade before threshing to dry seeds.
- Hand threshing is preferred to avoid damage to seeds during the threshing process. Small quantities of pods can be rubbed gently with the hands or on a rubber threshing pad to remove the seeds.
- For larger quantities of pods, thresh by placing them in cloth bags or sacks or spreading them on a canvas and beating with a rubber hose or stick.
-
When using mechanical threshers, carefully clean with a brush or air blower between lots to:
- Avoid contamination with seeds of accessions previously threshed; and
- Prevent diseases and pests from being passed from one accession to another.
- The cleaning should be done using air blowing machines or hand cleaning.
|
|
|
|
From uncleaned sesbania seeds (left) to clean sesbania seeds (right) (photos: ILRI) |
|
Visual inspection of seeds (final hand cleaning)
Visual inspection is essential in genebanks to reach high standards of removing debris, including empty or infected seeds, needed for long term storage. This is also important to check and prevent further spread of insect and fungal damage.
According to the International Seed Testing Association rules, the following steps should be followed:
- Spread the seeds on a flat well lit surface of contrasting color.
- An illuminated table can be used if available.
- Examine dry seeds with the naked eye or under a binocular microscope (this method reveals free moving insects, larva and eggs, mites, fungal fructifications, bacterial masses, infected plant debris).
- Examinations under or near ultraviolet reveals infections of certain fungi and bacteria through emission of fluorescence.
|
Light box used for visual inspection of seeds |
Separating seeds on a flat lit surface |
For insect and fungal damage
- Isolate the infested/infected samples.
- Destroy samples when quarantine diseases and/or insects are observed.
- Otherwise clean the seeds before storage using fumigation to control insects.
For mechanical damage and empty seeds
- Manually clean shriveled and damaged seeds.
|
|
Special treatments
- If infested with insects, place seeds in a deep freezer for 3 days.
- There is no information on the effects of agrochemicals on longevity during storage in genebanks, so it is better to avoid them. This is also better for staff health and safety.
- Fumigate with agrochemicals when the previous option is not possible.
Disposal of contaminated materials
- Incinerate or autoclave contaminated materials, to avoid spreading diseases and pests and destroy infected materials by burning.
Inspection and certification (purity analysis of seeds)
- This should be done according to International Seed Testing Association rules, following their standard methods (see purity analysis).
Recording information during seed cleaning
The following information should be recorded for each processing step:
- Accession number (an ID number).
- Lot number (an ID number).
- Seed weight (weight of seeds for storage).
- Reference to seed source (to trace the origin of the sample).
- Flags (Y/N) indicating completion of steps mentioned above (checking).
- Remarks.
Seed drying
Method recommended in genebank standards
- Use a dehumidified drying room with 13-15% relative humidity and 20-25oC.
- A relative humidity of 13-15% is however sometimes very hard to achieve. A relative humidity up to 25-30% can also be used, but it will prolong the drying period.
- The seed samples should be kept in paper, mesh or cotton bags on mesh racks during the drying period.
Drying time:
- Depending on seed size, from 2-8 weeks.
- The lower the relative humidity the faster the seeds will dry.
Moisture content before drying:
- Depending on seed size and seed coat, 8-12%.
- Up to 20% for Lupinus spp.
Moisture content for storage:
This is recommended in genebank standards (FAO/IPGRI, 1994) and experience shows that seeds remain with high viability for >20years, when using this standard.
- In general should be around 5% +/- 2%.
- Except for soybean that should be not less than 8%.
Critical moisture content:
The critical moisture content is the level below which further reduction in moisture content no longer increases seed longevity in low temperature storage. Critical moisture content values vary with storage temperature and species. For more information see Rao et al, 2006.
- It is recommended for most of the crops not to dry below 3% to avoid any adverse effects on the seed texture and viability. However, recent findings are suggesting drying to 2% to extend storage period.
-
It is recommended to dry to about 3-4% for some temperate forage legumes.

Seeds drying in a mesh bag (photo: ILRI)
Recording information during seed drying
The following information should be recorded for each processing step:
- Accession number (an ID number).
- Lot number (an ID number).
- Seed weight (weight of seeds for storage).
- Reference to seed source (to trace the origin of the sample).
- Flags (Y/N) indicating completion of steps mentioned above (checking).
- Remarks.
Determination of seed moisture content
Methods
Sampling frequency
|
Seed moisture determination (photo: ILRI) |
Standard drying periods under the drying conditions available in the genebank have been determined and so it is only necessary to measure moisture content on sample accessions once.
- Sample once at end of drying period to determine moisture for storage.
- Random sample during storage, in case there are holes in the bags.
Calculate seed moisture content by weight loss on a wet weight basis.
Sample size
This method follows a modified ISTA 2005 method using more replicates of smaller weight of seeds.
- Take 1.0-2.0g (0.25-0.5g for small seeded species) from each sample as two independent replicates.
Pre-drying
Pre-drying is obligatory if seeds are wet and their moisture content is suspected to be above 17% (10% for soybean and 13% for rice); it should be conducted prior to moisture content determination by oven-drying.
If pre-drying is required, proceed as follows:
- Weigh two sub-samples of 4-5g of seeds in their containers.
- Pre-dry the samples overnight in a warm, dry place such as a laboratory bench.
- Weigh them again in their containers and determine the loss of weight (loss of moisture) by subtraction.
-
Calculate the moisture content on a fresh-weight basis.
Grinding
Grinding is recommended for large seeded species (about 2-3 mm) to ensure small particles for efficient removal of moisture.
- Coarse grinding the sample using an adjustable mechanical grinder.
Species for which grinding is obligatory (ISTA, 2005):
|
Arachis hypogaea Avena spp. Cicer arietinum Citrullus lanatus Fagopyrum esculentum Glycine max |
Gossypium spp. Hordeum vulgare Lathyrus spp. Lupinus spp. Oryza sativa Phaseolus spp. |
Pisum sativum Secale cereale Sorghum spp. Triticum spp. Vicia spp. Zea mays |
Oven drying temperature
This is another standard and proven method, and the best practice is to follow the ISTA (2005) suggested methods.
Recording information during determination of seed moisture content
The following information must be recorded for each processing step:
- Accession number (an ID number).
- Lot number (ID number).
- Taxonomic identification.
- Date of test (the date that the test was commenced).
- Method (name of standard method).
- Drying temperature (oven temperature).
- Fresh weight in grams (weight of sample per replication).
- Dry weight in grams (weight of sample per replication).
- Moisture content (percentage moisture content after drying).
References and further reading
FAO/IPGRI. 1994. Genebank standards. Food and Agriculture Organization of the United Nations, Rome and International Plant Genetic Resources Institute, Rome. Available in English, Spanish, French and Arabic.
ISTA. 2008. International Rules for Seed Testing. International Seed Testing Association. ISTA secretariat, CH-Switzerland. Available from: www.seedtest.org/.
Rao NK, Hanson J, Dulloo ME, Ghosh K, Nowel D, Larinde M. 2006. Manual of seed handling in genebanks. Handbooks for Genebanks No. 8. Bioversity International, Rome, Italy. Available in English (1.5 MB), Spanish (1.4 MB) and French (1.9 MB).
More Articles...
Subcategories
-
main
- Article Count:
- 2
-
Conservation
- Article Count:
- 7
-
Regeneration
- Article Count:
- 1
-
Safety duplication
- Article Count:
- 1












