CGKB News and events Management strategies
Best practices for the safe transfer of maize germplasm
Contributors to this page: CIMMYT, Mexico (Etienne Duveiller, Monica Mezzalama, Eloise Phipps, Thomas Payne, Jesper Norgaard), Independent consultant (Jesse Dubin).
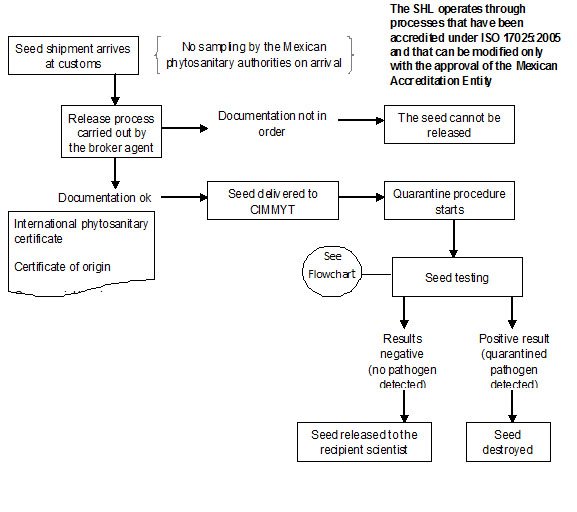
In CIMMYT Mexico, ‘Rules and Regulations for the Safe Movement of wheat and maize germplasm’ have been described by Dr. M. Mezzalama, Head of Seed Health Laboratory (SHL).
Since 1988 the CIMMYT SHL has been officially authorized by DGSV (Direccion General de Sanidad Vegetal) to carry out the quarantine procedures on seed introductions coming into Mexico and CIMMYT, and in April 2007 the SHL obtained accreditation under standard ISO/IEC 17025: 2005, “General requirements for testing and calibration laboratories”, as required by the Mexican government. An official DGSV inspector is assigned exclusively to CIMMYT to assist with thorough and timely seed inspection and importation.
All seed brought into CIMMYT, without exception, must be subjected to quarantine procedures in the Seed Health Laboratory.
The seed introduction procedure at CIMMYT Headquarters is summarized health flow chart
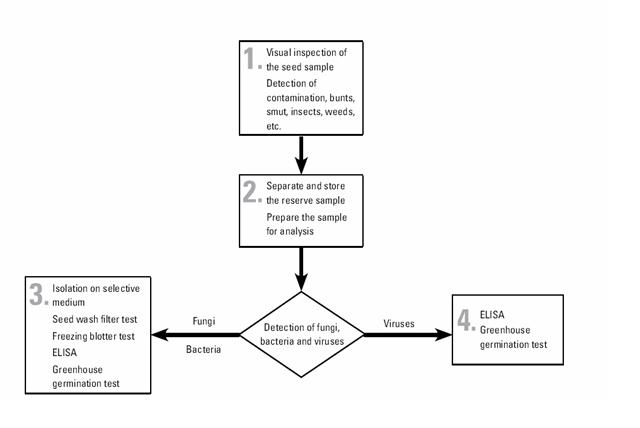
The pathogen detection and identification flowchart is described below:
All maize seed, both entering and leaving CIMMYT, must pass through the seed health laboratory. All seed undergoes the same set of testing procedures, although the key target pathogens vary between incoming and outgoing seed depending on relevant quarantine regulations.
CIMMYT generally uses well-established test procedures that may be found in any standard reference on seed health. The filter wash test is somewhat specialized, and the procedure are given in CIMMYT Seed Health Manual.
The tests used in CIMMYT are as follows:
- physical inspection for smut sori, nematode galls, ergot sclerotia, weed seeds, insect damage, etc.
- Seed wash filter test, which reveals the presence of fungal spores—including bunt teliospores (Tilletia spp.), smut spores (Urocystis and Ustilago spp.), and downy mildew oospores (Peronosclerospora and Sclerophthora spp.)—and of nematode cysts. This test takes around three hours, although large volumes of samples make take longer. Composite samples of outgoing seed may be used (with rechecking of individual lines in the event of a positive result).
- Freezing blotter test, which reveals the presence of imperfect fungi carried by seed and takes two weeks.
- Greenhouse germination test, for the expression, and thus detection, of seed-borne pathogens, and to check seed viability. This test takes three weeks. If symptoms appear on seedlings, further testing to identify the causal pathogen is carried out (i.e. ELISA or other tests).
- ELISA, or enzyme-linked immunosorbent assay, to detect specific bacteria and viruses. This test takes 24 hours.
- Downy mildew detection test. This test, with microscopic examination, detects Peronosclerospora and Sclerophthora spp on maize. It takes 24 hours.
Seed health testing on maize
|
Test
|
Pathogen type(s) detected
|
Pathogens of importance in incoming seed
|
Pathogens of quarantine importance in outgoing seed **
|
|
Seed wash filter test |
Fungi ( smuts) Nematodes |
Heterodera zeae* |
Ustilago maydis
|
|
Freezing blotter test
|
Imperfect fungi
|
Acremonium maydis*
|
Cochliobolus spp. Dilpodia spp. Fusarium spp. Lasiodiplodia theobromae |
|
Greenhouse germination test |
Bacteria
Viruses
|
Burkholderia andropogonis* Clavibacter michiganensis subsp. nebraskensis* Pantoea stewartii* Wheat streak mosaic virus*
|
Acidovorax aveanae subsp.avenae C. michiganensis subsp. nebraskensis P. stewartii Maize dwarf mosaic virus Maize chlorotic dwarf virus Sugarcane mosaic virus |
|
ELISA |
Bacteria
Viruses
|
Pantoea stewartii*
Wheat streak mosaic virus*
|
P. stewartii
Maize dwarf mosaic virus Maize chlorotic dwarf virus Sugarcane mosaic virus |
|
Downy mildew detection test |
|
Peronosclerospora maydis* P. philippinensis* P. sacchari* P. sorghi Sclerophthora rayssiae var. zeae* |
P. sorghi
|
* Quarantined under Norma Oficial Mexicana NOM-018-FITO-1995.
** According to information reported on importing countries requirements
References and further reading
Mezzalama M. 2012. Seed Health: Fostering the Safe Distribution of Maize and Wheat Seed: General guidelines. Third edition. Mexico, D.F.: CIMMYT. Available here (0,5 MB)
Warham EJ, Butler LD, Sutton BC. 1996. Seed Testing of Maize and Wheat: A Laboratory Guide. Mexico, D.F.: CIMMYT. 84 pp
Viruses - maize
Contributors to this section: CIMMYT, Mexico (Etienne Duveiller, Monica Mezzalama, Eloise Phipps), Independent consultant (Jesse Dubin).
|
Contents: |
Pathogen name
Maize dwarf mosaic virus (MDMV)
Importance
High economic importance, moderate phytosanitary importance.
Significance
Maize yield can be severely affected by MDMV. Yield losses of maize in the field can be as high as 40%. Moreover, in maize inbred lines or sweetcorn, especially after later sowing, yield can be reduced by 75% or more. Several crop loss studies in the USA, particularly with artificial inoculation, have reported yield reductions of 70 to 90%. In combination with maize chlorotic mottle virus, MDMV may also cause a severe reaction known as maize lethal necrosis.
Symptoms
Symptoms vary widely depending on host genotype and time of infection. Generally, infected plants develop a distinct mosaic—irregularities in the distribution of normal green color—typically appearing at the bases of the youngest leaves. There may also be narrow chlorotic streaks extending parallel to the veins. Later, the youngest leaves show a general chlorosis, and streaks are larger and more abundant. Reddish-violet discoloration and necrosis may also occur, particularly in sweetcorn and maize inbred lines. Any leaves that are already mature at the time of infection are not affected.
Infected plants are characterized by stunting (of variable severity) and shortening of upper internodes. Sterility is a common symptom of MDMV, often reaching 25% or more. In maize inbred lines, especially after an early infection, sterility can be much higher, causing complete or very high yield loss. Ear development can also be arrested, leading to incomplete grain filling. Plants infected early may produce nubbins or be totally barren. In severe cases the virus can cause premature plant death.
 Maize dwarf mosaic (photo: CIMMYT) |
Hosts
Major hosts: maize, Johnson grass (Sorghum halepense).
Minor hosts: oats (Avena sativa), millet (Panicum miliaceum), sugarcane (Saccharum officinarum).
Wild hosts: prairiegrass (Bromus catharticus), purpletop chloris (Chloris barbata), sour paspalum (Paspalum conjugatum), pearl millet (Pennisetum glaucum), Sudan grass (Sorghum sudanense), buffalo grass (Stenotaphrum secundatum), eastern gamagrass (Tripsacum dactyloides), marmalade grass (Urochloa plantaginea).
Geographic distribution
Worldwide.
Biology and transmission
Numerous aphid species (both adults and nymphs) transmit MDMV in a non-persistent manner. The virus is acquired by the aphid within ten seconds to two minutes of feeding and a latent period is not required. The virus overwinters in alternate hosts. The virus can be sap-transmitted under laboratory conditions. It is also transmitted by seed at low rates.
Detection/indexing methods used at CIMMYT
- Germination test on sterile soil.
- Detection with ELISA using AGDIA protocol (http://www.agdia.com).
Treatment/control
- Resistance, cultural practices, insecticides
Procedures followed in case of positive test used at CIMMYT
The seed lot is destroyed.
References and further reading
CAB International. 2007. Datasheet: Maize dwarf mosaic virus. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International.
CIMMYT. Maize Doctor information sheet: Maize dwarf mosaic virus. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=209. Extended information sheet: Maize dwarf mosaic virus. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=238. Date accessed 10 April 2010
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 38-40.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 94-95.
Pathogen name
Sugarcane mosaic virus (SCMV)
Importance
Moderate economic importance, low phytosanitary importance.
Significance
Yield losses due can be extensive, and in China the virus has been reported to seriously affect maize production. In Germany, SCMV was found to be more prevalent than MDMV, and to have a similar effect on growth and yield of maize. Early infection reduced plant height by 25%, total weight by 38%, and ear weight by 27%. In East Africa, inoculation with SCMV at the seedling stage caused yield losses of 18-46% in ten susceptible maize hybrids. Inoculation with the virus in Australia caused similar losses of up to 50%.
Symptoms
Symptoms vary widely depending on host genotype and time of infection. Generally, infected plants develop a distinct mosaic—irregularities in the distribution of normal green color—typically appearing at the bases of the youngest leaves. There may also be narrow chlorotic streaks extending parallel to the veins. Later, the youngest leaves show a general chlorosis, and streaks are larger and more abundant. Reddish-violet discoloration and necrosis may also occur, particularly in sweetcorn and maize inbred lines. Any leaves that are already mature at the time of infection are not affected.
Infected plants are characterized by stunting (of variable severity) and shortening of upper internodes. Sterility is a common symptom of MDMV, often reaching 25% or more. In maize inbred lines, especially after an early infection, sterility can be much higher, causing complete or very high yield loss. Ear development can also be arrested, leading to incomplete grain filling. Plants infected early may produce nubbins or be totally barren. In severe cases the virus can cause premature plant death.
 Sugarcane mosaic of maize (photo: CIMMYT) |
Hosts
Major hosts: maize, sugarcane (Saccharum officinarum), Johnson grass (Sorghum halepense).
Wild hosts: numerous grasses (Poaceae).
Geographic distribution
Worldwide.
Biology and transmission
Numerous aphid species (both adults and nymphs) transmit SCMV in a non persistent manner, with no latent period required. The virus overwinters in alternate hosts. It is also transmitted by seed at low rates.
Detection/indexing methods used at CIMMYT
- Germination test on sterile soil
- Detection with ELISA using AGDIA protocol (http://www.agdia.com).
Treatment/control
- Insecticides for aphid control
Procedures followed in case of positive test used at CIMMYT
- The seed lot is destroyed.
References and further reading
CAB International. 2007. Datasheet: Sugarcane mosaic virus. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International.
CIMMYT. Maize Doctor information sheet: MDMV/SCMV. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=209. Extended information sheet: MDMV/SCMV. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=238. Date accessed 10 April 2010
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 38-40.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 94-95.
Pathogen name
Maize chlorotic mottle virus (MCMV)
Importance
High economic importance, low phytosanitary importance.
Significance
The disease was first reported in Peru in 1973, where it can causes losses of 10-15% in some cultivars. Inoculation reduced yields by 50%. The combination of MCMV with maize dwarf mosaic virus or wheat streak mosaic virus may also cause a severe reaction known as maize lethal necrosis (MLN).
Symptoms
Fine chlorotic spots, appearing first on the youngest leaves, which coalesce and develop into broad chlorotic stripes along the veins. These chlorotic stripes contrast with dark green tissue when observed against the light. Affected leaves later die. Infected plants are stunted because of shortened internodes, and produce fewer and smaller ears. In most cases the tassel is malformed.
 Maize chlorotic mottle virus (MCMV)(photo: CIMMYT) |
Hosts
Maize, teosinte.
Geographic distribution
USA, Mexico, Argentina, Brazil, Peru.
Biology and transmission
The virus is transmitted mainly by several species of chysromelid leaf beetle, such as the corn flea beetle (Chaetocmena pulicaria), the cereal leaf beetle (Oulema melanopa), and corn rootworms (Diabrotica spp). The virus overwinters on crop residues; beetle larvae that feed on the residues in the absence of fresh material later transmit the virus to the new crop. Reports indicate that it is also transmitted at very low rates via infected seed.
Detection/indexing methods used at CIMMYT
- Germination test on sterile soil.
- Detection with ELISA using AGDIA protocol (http://www.agdia.com).
Treatment/control
- Insect control and cultural practices
Procedures followed in case of positive test used at CIMMYT
- The seed lot is destroyed.
References and further reading
CAB International. 2007. Datasheet: Maize chlorotic mottle virus. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International.
McGee DC. 1988. Maize Diseases: A Reference Source for Seed Technologists. St. Paul, MN: APS Press. pp. 124-125.
The CIMMYT Maize Program. 2004. Maize Diseases: A Guide for Field Identification. 4th Edition. Mexico, D.F.: CIMMYT. pp. 92-93.
Insects - maize
Contributors to this section: CIMMYT, Mexico (Etienne Duveiller, Monica Mezzalama, Eloise Phipps, Thomas Payne, Jesper Norgaard), Independent consultant (Jesse Dubin).
Khapra beetle
Importance
High economic and phytosanitary importance.
Significance
Trogoderma granarium is a general storage pest. It occurs mainly on cereals and cereal products, oilseeds (especially groundnuts and oilcakes), pulses and pulse products, and compound animal feeding stuffs. It is principally serious under hot dry conditions; complete destruction of grain and pulses may take place in a short time. In humid climates, it is not competitive with other storage pests with faster rates of increase.
Symptoms
Presence in stored grain. The stage most commonly seen during inspection is the larva, and the most usual evidence for infestation is cast larval skins.
 Trogoderma granarium (photo: EPPO) |
Hosts
Grain and stored product hosts.
The beetle is by nature an omnivorous protein scavenger and has been found in many locations that would not be obvious food sources.
Geographic distribution
Trogoderma granarium is established within an area roughly limited by the 35° parallel to the north, the Equator to the south, West Africa to the west and Myanmar to the east; i.e. the warm dry regions along the Suez route from the Indian subcontinent to Europe. T. granarium has been introduced into other areas with similar climatic conditions, especially the alternative route between India and Europe around Africa. Initially, these introductions caused severe damage but outbreaks have been local and most have been eradicated. T. granarium has also established in some areas with unfavorable climates, in protected storage environments only, for example in Western Europe and Japan.
EPPO distribution map: http://www.eppo.org/QUARANTINE/insects/Trogoderma_granarium/TROGGA_map.htm
Biology and transmission
The natural spread of this pest is limited. International spread is mainly by larvae in commodities, empty sacks, and in the structure of ships and dry cargo containers. Second-hand bags are also a means of spread.
Detection/indexing methods used at CIMMYT
- Physical inspection of seed.
Treatment/control
- Fumigation
Procedures followed in case of positive test used at CIMMYT
- The seed lot is destroyed (the insect is quarantined in Mexico).
EPPO protocols
EPPO Diagnostic Protocols for Regulated Pests PM 7/13(1): Trogoderma granarium. EPPO Bulletin 32: 241-243. [online] Available from URL: http://www.eppo.org/QUARANTINE/insects/Trogoderma_granarium/TROGGA_protocol.pdf. Date accessed 10 April 2010
References and futher reading
CAB International. 2007. Datasheet: Trogoderma granarium. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International.
EPPO. Data Sheets on Quarantine Pests: Trogoderma granarium. [online] Available from URL: http://www.eppo.org/QUARANTINE/insects/Trogoderma_granarium/TROGGA_ds.pdf. Date accessed 10 April 2010
Larger grain borer
Importance
High economic and phytosanitary importance.
Significance
Infestations in maize may start on the mature crop in the field, i.e. when moisture content is at or below 18%. Weight losses of up to 40% have been recorded in Nicaragua from maize cobs stored on the farm for 6 months. In Tanzania, up to 34% losses have been observed after 3 months storage on the farm, with an average loss of 8.7%.
Symptoms
These insects infest both stored grain and maturing maize ears in the field. In a very short time, the adults produce large quantities of floury dust as they bore into and feed on the grains. Damaged grains can readily be identified since they are usually covered by a film of this dust.
The adult beetles are 3 to 5mm long, cylindrical in shape and reddish brown to dark brown. Their heads are turned down and appear to be covered by a hood. They are able to fly.
 Prostephanus truncates |
Hosts
Major hosts: maize, cassava (Manihot esculenta), dried stored products, wood.
Minor hosts: Dioscorea (yam), Sorghum bicolor (sorghum), triticale, wheat.
Geographic distribution
P. truncatus is indigenous in Central America, tropical South America, and the extreme south of the USA as a major, but localized, pest of farm-stored maize. It was introduced into Tanzania, probably in the late 1970s, and has become a serious pest of stored maize and dried cassava in that part of East Africa; it has since spread into Kenya, Burundi, Rwanda, Malawi, Zambia, Mozambique, Namibia and South Africa, and is almost certainly present but unreported in several other countries in the region. It was first found in West Africa in Togo in 1984 and it has since spread to Benin, Nigeria, Ghana, Niger and Burkina Faso. A separate outbreak occurred in Guinea in Conakry.>/p>
Biology and transmission
P. truncatus may be attracted to maize grain and dried cassava over short distances. However, field studies in both Mexico and Togo suggest that there is no long-range attraction of adult P. truncatus to maize grain or cobs, or dried cassava; this is not surprising because wood is the major host of this beetle.
Adults frequently initiate their attack on stored maize cobs with intact sheaths by boring into the base of the maize cob core, eventually gaining access to the grain via the apex of the cob by crawling between the sheathing leaves. Adults bore into the maize grains, making neat round holes, and as they tunnel from grain to grain they generate large quantities of maize dust. Adult females lay eggs in chambers bored at right angles to the main tunnels. Egg-laying on stabilized grain, like that on the maize cob, is more productive than on loose-shelled grain as the oviposition period is longer, equal in length to the life of the female, and the eggs are laid at a greater rate.
Larvae hatch from the eggs after about three days at 27°C and seem to thrive on the dust produced by boring adults. For example, large numbers of larvae develop and pupate in dust at the base of dense laboratory cultures. Larvae may also crawl into and feed on slightly damaged kernels. Pupation takes place inside the kernels, and emerging adults then cut their way out of the kernels.
The success of this pest may be partly due to its ability to develop in grain in low moisture conditions, under which many other storage pests are unable to multiply.. For example, Sitophilus oryzae, a species occurring in a very similar ecological niche, needs a grain moisture content of at least 10.5% for development. Thus, in dry conditions, P. truncatus probably benefits from the absence of any significant competition from other storage pests.
Detection/indexing methods used at CIMMYT
- Physical inspection of seed.
Treatment/control
- Fumigation; resistance
Procedures following in case of positive test used at CIMMYT
- The seed lot is destroyed.
References and further reading
CAB International. 2007. Datasheet: Prostephanus truncatus. In Crop Protection Compendium, 2007 Edition. Published as CD-ROM and website. Wallingford, UK: CAB International.
CIMMYT. Maize Doctor information sheet: Grain borers. [online] Available from URL:http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=221. Extended information sheet: Grain borers. [online] Available from URL: http://maizedoctor.cimmyt.org/index.php?option=com_content&task=view&id=329. Date accessed 10 April 2010
Safe transfer of chickpea germplasm
Contributors to this page: ICRISAT, India (RP Thakur, AG Girish, VP Rao); ICARDA, Syria (Siham Asaad, Abdulrahman Moukahal).
The Plant Quarantine Laboratory (This email address is being protected from spambots. You need JavaScript enabled to view it.) at ICRISAT, Patancheru, India follows the plant quarantine requirements of the ICRISAT scientific community for the germplasm exchange of ICRISAT’s mandate crops: sorghum [Sorghum bicolor (L.) Moench)], pearl millet [Pennisetum glaucum (L.) R. Br.], chickpea [Cicer arietinum (L.)], pigeonpea [Cajanus cajan (L.) Millspaugh], groundnut [Arachis hypogaea (L.)] and six small millets: finger millet [Eleusine coracana (L.) Gaertn.], foxtail millet [Setaria italica (L.) Beauv.], little millet (Panicum sumatrense Roth.ex Roem. & Schult.), barnyard millet [Echinochloa crussgalli (L.) Beauv.], proso millet [Panicum miliaceum (L.)] and kodo millet [Paspalum scrobiculatum (L.)].
Indian plant quarantine regulations are legislated under the Destructive Insects and Pests Act, 1914 and the Plant Quarantine Order 2003 for the purpose of prohibiting and regulating the import of agricultural articles into India.
- Seed and plant material of these crops cannot be exported/ imported directly by the institute’s scientists.
- The National Bureau of Plant Genetic Resources (NBPGR) of the Indian Council of Agricultural Research (ICAR), New Delhi, is the plant quarantine authority responsible for ICRISAT’s germplasm exchange.
In 1985, NBPGR established a Regional Station at Rajendranagar, Hyderabad to implement the quarantine regulations in South India, to ensure the safe movement of germplasm.
Within the CGIAR, both ICRISAT and ICARDA are responsible for chickpea and the information on quarantine regulations and guidelines is presented for both institutions.
This page includes sections on:
- Import and export requirements.
- Technical guidelines for the safe movement of germplasm and detection of relevant pathogens and pests.
- Best practices in place at ICRISAT.
References and further reading
Ahmed KM, Ravinder Reddy Ch.1993. A pictorial guide to the identification of seedborne fungi of sorghum, pearl millet, finger millet, chickpea, pigeonpea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the semi-Arid Tropics. 200 pp.
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
More Articles...
Subcategories
-
main
- Article Count:
- 11
-
Stog
- Article Count:
- 2
-
Stog-rice
- Article Count:
- 7
-
Stog-sorghum
- Article Count:
- 11
-
Stog-common-bean
- Article Count:
- 10
-
stog-forage-legume
- Article Count:
- 10
-
stog-forage-grass
- Article Count:
- 11
-
stog-maize
- Article Count:
- 9
-
stog-chickpea
- Article Count:
- 10
-
stog-millets
- Article Count:
- 12
-
stog-barley
- Article Count:
- 10
-
stog-groundnut
- Article Count:
- 9
-
stog-pigeon-pea
- Article Count:
- 8
-
stog-wheat
- Article Count:
- 10
-
stog-lentil
- Article Count:
- 9
-
stog-cowpea
- Article Count:
- 10
-
stog-faba-bean
- Article Count:
- 9
-
risk management
- Article Count:
- 4
-
decision support tool
- Article Count:
- 3
-
stog-clonal
- Article Count:
- 23
-
developing strategies
- Article Count:
- 4




