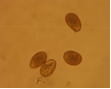
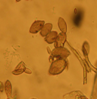
CGKB News and events stog-cowpea
Viruses - cowpea
Contributors to this section: IITA, Nigeria (M. Ayodele, L. Kumar).
|
Contents: |
Scientific name
Cowpea mosaic virus (CpMV) (genus Comovirus)
Other scientific name
Cowpea mosaic comovirus
Importance
High
Significance
Not reported.
Symptoms
Not reported.
Hosts
Reported hosts are Vigna unguiculata, Glycine max , (soyabean)Vigna umbellata , (Rice- bean)
Geographic distribution
India, Pakistan, Nigeria, Togo
Biology and transmission
Not reported.
Detection/indexing methods used at IITA
- A combination of methods are used for detection and identification
- Methods based on biological properties of the virus
- Growing out test : Symptomatology. Symptoms used to characterized the viruses
- Visual inspection : This requires expertise and adequate field experience and confirmation using confirmatory tests
- Transmission tests : using indicator plants: mechanical sap, grafting, vector
- Physical: Electron microscopy, Serology(ELISA and PCR)
Treatment/control
Strategies for treatment are directed towards prevention of virus infection which are:
- Planting virus free materials
- Controlling vectors where applicable
- No imports of germplasm from countries known to harbour the virus
- Host plant resistance
- Plants with symptoms are rogued during active growth
Procedures in case of positive test
- At IITA, all lines testing positive are discarded after different diagnostic tests have been conducted. There are no economic chemical agents effective against these plant viruses. Hot water is sometimes used, but most of the time when used as seed treatment, the virus is not eliminated from the seeds and the seed quality is reduced.
- If germplasm material is valuable, for import / export,the seeds are grown under containment, inspection during active growth, rogue plants with symptoms and incinerate.
- Serological testing of symptomless lines by ELISA and PCR
- Harvested lines found free from the virus are released to breeders and / or recommended for international distribution.
References and further reading
Naidu RA, d’Hughes J. 2001, Methods for the detection of plant viruses. In Proceedings of the Plant Virology in Sub- Saharan Africa. Conference organized by IITA, 4-6 June 2001.
Scientific name
Blackeye cowpea mosaic virus (BICMV) (genus Potyvirus)
Other scientific name
Bean common mosaic virus strain blackeye cowpea
Importance
High
Significance
Yield reduction from expected 2500kg/ha to 50kg/ha was reported in fields infected with BlCMV in India (Puttaraju et al. (2000)
Cowpea varieties inoculated with BlCMV at the primary leaf stage showed 92-100% infection at first trifoliate leaf.
Iizuka (1990) reported that in field trials, the virus reduced yield of adzuki beans (Vigna angularis) by 33%.
Symptoms
Growth stages and plant parts affected by the virus are seedling, and vegetative stages, the leaves and the whole plant.
Leaves: discoloration, mosaic, mottling, vein banding, vein chlorosis, vein yellowing, leaf deformation and yellow spots.
Seeds : shrivelling
Whole plant: severe green and yellow mosaic, vein banding, mottle, blistering, leaf roll and growth reduction
Presence of virus in the cotyledons, and embryo axes in mature cowpea seeds was reported by Provvidenti, 1986). Sekar and Sulochana (1988)
The spread of BICMV in the fields is effected by aphids after initial seed transmission from infected seeds planted.
Hosts
Reported major hosts areVigna unguiculata ,, Arachis hypogaea ,, Glycine max , Vigna angularis , Vigna mungo , Vigna radiata , , Voandzeia subterranea , (bambara groundnut)(mung bean)(black gram)(adzuki bean)(soyabean)(groundnut)(cowpea) Desmodium incanum, D. tortuosum and Sphenostylis stenocarpa have also been reported as natural hosts. Taiwo et al ( 1982), Brunt et al. (1990), Zhao et al. (1991b), Fery and Dukes (1992).
Geographic distribution
Cosmopolitan.
Biology and transmission
The virus is vector transmitted, mostly by aphids in a non-persistent manner. From experimental results some aphid species have been found to be vectors of the virus such as Aphis craccivora (Bashir and Hampton, 1994, Aphis gossypii ( Mali et al., 1988)
Macrosiphum euphorbiae Murphy et al., 1987), and Myzus persicae (../Jesse Consult Mexico Nov/RefPtr=3N4ceb); Mali et al., 1988].
The virus is seed borne and seed transmitted (Tsuchizaki et al., 1986). Dijkstra et al., 1987
Seedborne infection of the virus has been detected with incidences as high as 50% in cowpea seed by Zettler and Evans, 1972; and Gillaspie et al., 1993.
Detection/indexing methods
- A combination of methods is used for detection and identification
- Methods based on biological properties of the virus
- Growing out test : Symptomathology . Symptoms used to characterized the viruses
- Visual inspection, symptoms This requires expertise and adequate field experience and confirmation using confirmatory tests
- Transmission tests : using indicator plants: mechanical sap, grafting, vector
- Physical: Electron microscopy, Serology(ELISA and PCR).
Treatment/control
Strategies for treatment are directed towards prevention of virus infection which are:
- Planting virus free materials
- Controlling vectors where applicable
- No imports of germplasm from countries known to harbour the virus/ additional declaration that seeds for international distribution were grown in locations known to be free of the virus
- Imports subjected to post entry processing on arrival
- Host plant resistance
- Plants with symptoms are rogued during active growth
Procedures in case of positive test at IITA
- All lines testing positive are discarded after different confirmatory diagnostic tests have been conducted. Hot water has been recommended, but most of the time when used for seed treatment, the seeds deteriorate and the viuruses not eliminated
- If germplasm material is valuable, grow import / export seeds under containment, inspection during active growth, rogue plants with symptoms
- Serological testing of symptomless lines
- Harvest lines found free from the virus and release to breeders and / or recommend for international distribution
References and further reading
Bashir M, Hampton RO. 1994. Seed and aphid transmission of some isolates of blackeye cowpea and cowpea aphid-borne mosaic potyviruses. Pakistan Journal of Phytopathology, 6(2):140-146.
Brunt A, Crabtree K, Gibbs A. 1990. Viruses of Tropical Plants. Wallingford, UK: CAB International
Dijkstra J, Bos L, Bouwmeester HJ, Hadiastono T, Lohuis H. 1987. Identification of blackeye cowpea mosaic virus from germplasm of yard-long bean and from soybean, and the relationships between blackeye cowpea mosaic virus and cowpea aphid-borne mosaic virus. Netherlands Journal of Plant Pathology, 93(3):115-133.
Fery RL, Dukes PD. 1992. 'Carolina Crowder' southernpea. HortScience, 27(12):1335-1337.
Gillaspie AGJr, Hopkins MS, Pinnow DL. 1993. Relationship of cowpea seed-part infection and seed transmission of blackeye cowpea mosaic potyvirus in cowpea. Plant Disease, 77(9):875-877
Iizuka N. 1990. Studies on virus diseases of adzuki bean (Vigna angularis Wight) in Japan. Bulletin of the Tohoku National Agricultural Experiment Station, No. 82:77-113
Mali VR, Mundhe GE, Patil NS, Kulthe KS. 1988. Detection and identification of blackeye cowpea mosaic and cowpea aphid borne mosaic viruses in India. International Journal of Tropical Plant Diseases, 6(2):159-173.
Murphy JF, Barnett OW, Witcher W. 1987. Characterization of a blackeye cowpea mosaic virus strain from South Carolina. Plant Disease, 71(3):243-248.
Provvidenti R. 1986. Seed transmission of blackeye cowpea mosaic virus in Vigna mungo. Plant Disease, 70(10):981
Puttaraju HR, Prakash HS, Shetty HS. 2000. Field incidence, seed-transmission and susceptibility of cowpea varieties with reference to Blackeye Cowpea Mosaic Potyvirus. Seed Research, 28(2):196-202
Sekar R, Sulochana CB. 1988. Seed transmission of blackeye cowpea mosaic virus in two cowpea varieties. Current Science, 57(1):37-38
Taiwo MA, Gonsalves D, Provvidenti R, Thurston HD. 1982. Partial characterization and grouping of isolates of blackeye cowpea mosaic and cowpea aphidborne mosaic viruses. Phytopathology, 72(6):590-596.
Tsuchizaki T, Senboku T, Iwaki M, Kiratiya-Angul S, Srithongchai W, Deema N, Ong CA. 1986. Blackeye cowpea mosaic virus from asparagus bean (Vigna sesquipedalis) in Thailand and Malaysia. Technical Bulletin of the Tropical Agriculture Research Center, No.21:213-218.
Zettler FW, Evans IR. 1972. Blackeye cowpea mosaic virus in Florida: host range and incidence in certified cowpea seed. Proceedings of the Florida State Horticultural Society, 85:99-101.
Scientific name
Cowpea severe mosaic virus (CpSMV) (genus Comovirus)
Other scientific names
Cowpea severe mosaic comovirus, Puerto Rico cowpea mosaic virus
Importance
High
Significance
Yield losses of 60-80% caused by CSMV were reported in Brazil
Symptoms
Not reported.
 Cowpea severe mosaic virus (photo: IITA) |
Hosts
The major hosts of this virus are Vigna unguiculata (cowpea) Canavalia ensiformis (gotani bean), Crotalaria juncea (sunn hemp), Glycine max (soyabean), Phaseolus vulgaris (common bean), Psophocarpus tetragonolobus (winged bean), Vigna radiata (mung bean).
Geographic distribution
Widespread in North , South and Central America, Brazil, Mexico, Peru, Cuba, Trinidad and Tobago, Pakistan, Senegal, Nigeria.
Biology and transmission
Seed borne, sap and insect transmitted mostly beetles such as Cerotoma sp.
Detection/indexing methods at IITA
- A combination of methods is used for detection and identification
- Methods based on biological properties of the virus
- Growing out test : Symptomathology . Symptoms used to characterized the viruses
- Visual inspection :This requires expertise and adequate field experience and confirmation using confirmatory tests
- Transmission tests : using indicator plants: mechanical sap, grafting, vector
- Physical: Electron microscopy, Serology(ELISA and PCR).
Treatment/control
- Planting virus free materials
- Controlling vectors where applicable
- No imports of germplasm from countries known to harbour the virus/ additional declaration that seeds for international distribution were grown in locations known to be free of the virus
- Imports subjected to post entry processing on arrival
- Host plant resistance
- Field inspection
- Rogue plants with symptoms and discard
Procedures in case of positive test at IITA
All lines testing positive are discarded after different confirmatory diagnostic tests have been conducted
For import
- If germplasm material is valuable, grow import material under containment, inspection during active growth, rogue plants with symptoms
- Serological testing of symptomless lines
- Harvest lines found free from the virus and release to breeders for research.
For export
- Seeds from symptomless lines harvested from the multiplication plots meant for international distribution are grown in the screen houses.
- The lines are inspected during active growth in collaboration with Plant Quarantine officials. Plants with symptoms are rougued and incinerated. Leaf samples and seeds from symptomless plants are tested serologically by ELISA or PCR
- Only disease free lines are distributed internationally
References and further reading
Alconero R, Santiago. (1973). Phytopathology 63, 120-123
Diaz A. (1974). Phytopathology 64, 767.
Lima JAA, Nelson MR. (1977). Pl. Dis. Reptr. 61, 864-867
Shepherd RJ. (1964). Phytopathology 54, 466-473
Scientific name
Cowpea aphid-borne mosaic virus (CAMV, CABMV) (genus Potyvirus)
Other scientific names
Cowpea Moroccan aphid-borne mosaic virus (Fischer & Lockhart, 1976)
Importance
High
Significance
Complete loss of a cowpea crop in northern Nigeria resulting from CABMV attack under irrigated field conditions was reported by Raheja and Leleji (1974). A yield loss of 13-87% due to natural infection of cowpea by CABMV was reported in Iran by Kaiser and Mossahebi, ( 1975). While Kannaiyan and Haciwa, (1993) reported a loss 48-60% in Zambia).
Symptoms
All the plant stages and parts are affected: flowering , fruiting, seedling and vegetative stages in addition to the pods, growing points, inflorescence, leaves, seeds, stems and whole plant.Symptoms vary according to the cowpea cultivar and the existing CABMV race. Shoyinka et al., (1997) reported that CABMV symptoms observed on cowpea under field conditions were extremely variable.
Symptoms expressed on the different parts are:
-
Leaves: primary leaves show vein-clearing, vein-yellowing, diffused chlorotic spots / patches, or an intense chlorosis (Phatak, 1974; Bashir, 1992).
-
On trifoliate leaves: vein-yellowing, yellow mosaic with or without dark-green, irregular vein-banding and blistering, deformation, puckering and stunting (Kaiser and Mossahebi, 1975), mosaic pattern, cupping ,distortion and necrotic lesions. Williams (1975)
-
Inflorescence: lesions, virus in the pollens, anthers and ovaries, the plumule, and cotyledons, Phatak (1974), Tsuchizaki et al. (1970)
-
Growing points: lesions; abnormal forms
-
Stem: necrosis , abnormal forms and growth.
-
Pods: deformation , lesions; discoloration
-
Seeds: reduction in seed size, discoloration, lesion and loss of viability (Kaiser and Mossahebi, 1975).
-
Whole plant: Systemic mosaic, stunting, distortion; rosetting
|
Cowpea aphid-borne mosaic virus (photo: IITA) |
Hosts
Although cowpea (Vigna unguiculata ) is the main host of this virus other major hosts include, Sesamum indicum (sesame ),Voandzeia subterranea . While (bambara groundnut)Glycine max , (soyabean)Pachyrhizus erosus , (yam bean)Pisum sativum , (pea)Vigna radiata are reported as minor hosts.(mung bean)
Glycine max, Cajanus cajan, Cicer arietinum, Lablab purpureus, Vigna subterranea and Lens culinaris were reported as symptomless carriers by Mazyad et al.,( 1981)
Edwardson and Christie (1986) reported that CABMV infected 53 species in 28 genera of the Leguminosae. The virus is also said to infect 13 other families among which are Amaranthaceae, Aizcaceae, Chenopodiaceae, Cucurbitaceae, Hydrophyllaceae, Labiatae, Iridaceae, Leguminosae, Scrophulariaceae, Polygonaceae, Solanaceae and Pedaliaceae. Isolates from the different parts of the world have different hosts range (Bock, 1973).
Geographic distribution
The virus is worlwide in distribution. It is considered to be a major and widespread disease of cowpea through out sub-Saharan Africa (Bock, 1973; Ladipo, 1976; Thottappilly and Rossel, 1985; Burke et al., 1986
The virus has also been reported in Europe, Asia, Africa, Brazil, USA, Australia and Papua New Guinea
Biology and transmission
CABMV is seed borne and seed transmitted, (Allen, 1983; Rossel and Thottappilly, 1990). The virus survives in infected seed, volunteer host plants and in viruliferous aphids. Thottappilly, (1992)
The virus is transmitted mechanically ( sap), and vector transmitted by several aphid species in a stylet-borne non-persistent manner, and Aphis craccivora is the most efficient vector (Bock, 1973;Atiri et al., 1984, 1986). The aphid species reported to be vectors of CABMV in addition to Aphis craccivora, are A. gossypii, A. spiraecola, A. medicaginis, A. fabae, A. citricola, A. sesbaniae, Macrosiphum euphorbiae, Myzus persicae, Rhopalosiphum maidis, Cerataphis palmae and Acyrthosiphon Dijkstra et al., 1987; Mali et al., 1988; Thottapilly, 1992; Thottapilly and Rossel, 1992; Roberts et al., 1993; Bashir and Hampton, 1994).
Detection/indexing methods at IITA
- Growing out test: in screen houses/ containment facility to determine presence/ absence of virus symptoms in the seedlings growing from the virus-infected seeds.
- Infectivity test: presence of virus assayed by inoculating extracts of seed or seedlings on to indicator hosts under containment facility
- Serological tests: most reliable and effective methods for the detection of seedborne viruses and virus from plant tissues
- ELISA: Enzyme-linked immunosorbent assay. PCR
Treatment/control
- No seed treatment has yet been reported to eliminate CABMV directly from seed
- IITA has resistant cowpea lines
- Grow resistant cowpea cultivars against aphids attack and CABMV infection
- Production of virus-free seed to supply to breeders.
- Production of seeds for international distribution under certification scheme
- Field inspection during active growth and roguing of diseased plants
Procedures in case of positive test at IITA
All lines testing positive are discarded after different confirmatory diagnostic tests have been conducted.
For Import:
- If germplasm material is valuable, grow import material under containment, inspection during active growth, rogue plants with symptoms
- Serological testing of symptomless lines
- Harvest lines found free from the virus and release to breeders for research
For export:
- Seeds from symptomless lines harvested from the multiplication plots meant for international distribution are grown in the screen houses.
- The lines are inspected during active growth in collaboration with Plant Quarantine officials. Plants with symptoms are rougued and incinerated. Leaf samples and seeds from symptomless plants are tested serologically by ELISA or PCR
- Only disease free lines are distributed internationally
References and further reading
Allen DJ. 1983. Disease resistance in crop improvement. In: The Pathology of Tropical Food Legumes. Chichester, UK: John Wiley and Sons, 210-213.
Atiri GI, Ekpo EJA, Thottappilly G. 1984. The effect of aphid-resistance in cowpea on infestation and development of Aphis craccivora and the transmission of cowpea aphid-borne mosaic virus. Annals of Applied Biology, 104(2):339-346.
Atiri GI, Enobackhare DA, Thottappilly G. 1986. The importance of colonizing and non-colonizing aphid vectors in the spread of cowpea aphid-borne mosaic virus in cowpea. Crop Protection, 5(6):406-410
Bashir M. 1992. Serological and biological characterization of seed-borne isolates of blackeye cowpea mosaic and cowpea aphid-borne mosaic potyviruses in Vigna unguiculata (L.) Walp. PhD Thesis, Oregon State University, Corvallis, Oregon, USA.
Bashir M, Hampton RO. 1994. Seed and aphid transmission of some isolates of blackeye cowpea and cowpea aphid-borne mosaic potyviruses. Pakistan Journal of Phytopathology, 6(2):140-146
Bock KR. 1973. East African strains of cowpea aphid-borne mosaic virus. Annals of Applied Biology, 74(1):75-83;
Burke DW, Ditshipi P, DeMooy CJ. 1986. Virus diseases of cowpeas in dryland and irrigated plots in Botswana. Plant Disease, 70(8):801
Dijkstra J, Bos L, Bouwmeester HJ, Hadiastono T, Lohuis H. 1987. Identification of blackeye cowpea mosaic virus from germplasm of yard-long bean and from soybean, and the relationships between blackeye cowpea mosaic virus and cowpea aphid-borne mosaic virus. Netherlands Journal of Plant Pathology, 93(3):115-133
Edwardson JR, Christie RG. 1986. Viruses infecting forage legumes. Vol. II. Monograph No. 14. Agriculture Experimental Station, University of Florida, Gainesville, Florida, USA
Kaiser WJ, Mossahebi GH. 1975. Studies with cowpea aphid-borne mosaic virus and its effect on cowpea in Iran. Plant Protection Bulletin, FAO, 23(2):33-39
Kannaiyan J. Haciwa HC. 1993. Diseases of food legume crops for the scope of their management in Zambia. FAO Plant Protection Bulletin, 41:73-90.
Ladipo JL. 1976. A vein-banding strain of cowpea aphid-borne mosaic virus in Nigeria. Nigerian Journal of Science, 10:77-86.
Mali VR, Mundhe GE, Patil NS, Kulthe KS. 1988. Detection and identification of blackeye cowpea mosaic and cowpea aphid borne mosaic viruses in India. International Journal of Tropical Plant Diseases, 6(2):159-173
Mazyad HM, El-Hammady M, El-Amrety AA, El-Din ASG. 1981. Studies in cowpea aphid-borne mosaic virus in Egypt. Agricultural Research Review, 59(2):167-178
Phatak HC. 1974. Seed-borne plant viruses - Identification and diagnosis in seed health testing. Seed Science and Technology, 2:3-155.
Raheja AK, Leleji OI. 1974. An aphid-borne virus disease of irrigated cowpea in Northern Nigeria. Plant Disease Reporter, 58(12):1080-1084
Roberts JMF, Thottappilly G, Hodgson CJ. 1993. The ability of Aphis craccivora, A. gossypii and A. citricola to transmit single and mixed viruses to cowpeas. Journal of Phytopathology, 138(2):164-170
Rossel HW, Thottappilly G. 1990. Possible dependence of geographical distribution of virus diseases of cowpea in African agroecological parameters. In: Allen DJ, ed. Proceedings of Working Group Meeting on Virus Diseases of Beans and Cowpeas in Africa. CIAT Africa Workshop Series No. 13. Cali, Colombia: Centro Internacional de Agricultura Tropical, 33-37.
Shoyinka SA, Thottappilly G, Adebayo GG, Anno-Nyako FO. 1997. Survey on cowpea virus incidence and distribution in Nigeria. International Journal of Pest Management, 43(2):127-132
Thottappilly G, Rossel HW. 1985. Worldwide occurrence and distribution of virus diseases, In: Singh SR., Rachie RO, eds. Cowpea Research, Production and Utilization. Chichester, UK: John Wiley and Sons, 155-171.
Thottappilly G, Rossel HW. 1992. Virus diseases of cowpea in tropical Africa. Tropical Pest Management, 38(4):337-348
Tsuchizaki T, Yora K, Asuyama H. 1970. The viruses causing mosaic of cowpea and azuki bean, and their transmissibility through seeds. Annals of the Phytopathological Society of Japan, 36:112-120.
Scientific name
Cowpea mottle virus (CPMoV) (genus Carmovirus)
Importance
High
Significance
The impact of infection on the yield of cowpea is not known but yield losses of 64-80% in groundnuts were reported in Kenya by Bock et al., 1976, 1977). Other unquantified yield losses, of groundnuts, soyabeans, bambara groundnuts (Vigna subterranea) and winged beans (Psophocarpus tetragonolobus ) have been reported by Fauquet et al., (1979); Fortuner et al., (1979); Dubern and Dollet, (1981); Thouvenel et al., (1982); Fauquet and Thouvenel, (1987); Saleh et al., (1989); and Reddy, (1991).
Symptoms
The virus affects all growing stages of the plant, flowering, podding, and seedling stages. The leaves and the whole plant are also infected.
Symptoms exhibited on cowpea are :
Leaves: mild to severe chlorotic mottling, distortion, stunting.
Whole plants: stunting.
|
|
|
|
|
Cowpea mottle virus (photos: IITA) |
||
Hosts
Although the natural hosts of CPMV are leguminous species, the virus also occurs naturally in tomatoes in Israel and Nigeria.
The major hosts are: Vigna unguiculata (cowpea), Arachis hypogaea (groundnut), Glycine max (soyabean), Lycopersicon esculentum (tomato), Phaseolus vulgaris (common bean).
The minor recorded hosts are Calopogonium mucunoides (calopo (Australia)), Mucuna pruriens (Buffalobean), Phaseolus lunatus (lima bean), Phaseolus radiata, Psophocarpus tetragonolobus (winged bean), Vicia faba (broad bean), Voandzeia subterranea (bambara groundnut) while Centrosema pubescens (Centro), Desmodium tortuosum (Florida beggarweed), Stylosanthes gracile , Tephrosia villosa are reported as wild hosts
Geographic distribution
The virus is widely distributed in Africa, Asia, Oceania and South America.
Biology and transmission
Transmitted by whiteflies Bemisia tabaci,(Brunt, 1995), Thottappilly and Rossel, 1992). Seed borne and seed transmitted, (Brunt and Kenten, 1973), (Nain et al., 1994).
Detection/indexing methods at IITA
A combination of methods is used for detection and identification. The methods are based on biological properties of the virus.
- Growing out test : Symptomathology . Symptoms used to characterize the viruses
- Visual inspection: this requires expertise and adequate field experience and confirmation using confirmatory tests
- Transmission tests : using indicator plants: mechanical sap, grafting, vector
- Physical: Electron microscopy, Serology(ELISA and PCR)
Treatment/control
- No seed treatment has yet been reported to eliminate CABMV directly from seed
- IITA has resistant cowpea lines
- Grow resistant cowpea cultivars against aphids attack and CABMV infection
- Production of virus-free seed to supply to breeders.
- Production of seeds for international distribution under certification scheme
- Field inspection during active growth and roguing of diseased plants
Procedures in case of positive test at IITA
All lines testing positive are discarded after different confirmatory diagnostic tests have been conducted.
For Import :
- If germplasm material is valuable, grow import material under containment, inspection during active growth, rogue plants with symptoms
- Serological testing of symptomless lines
- Harvest lines found free from the virus and release to breeders for research.
For export:
- Seeds from symptomless lines harvested from the multiplication plots meant for international distribution are grown in the screen houses.
- The lines are inspected during active growth in collaboration with Plant Quarantine officials. Plants with symptoms are rougued and incinerated. Leaf samples and seeds from symptomless plants are tested serologically by ELISA or PCR
- Only disease free lines are distributed internationally
References and further reading
Bock KR, Guthrie EJ, Meredith G, Njuguna JGM. 1976. Plant pathology: Groundnut viruses. Report of the East African Agriculture and Forestry Research Organisation for 1974. Nairobi, Kenya: East African Agriculture and Forestry Research Organisation, 120-128.
Bock KR, Guthrie EJ, Meredith G, Njuguna JGM. 1977. Plant pathology. Report of the East African Agriculture and Forestry Research Organisation for 1975. Nairobi, Kenya: East African Agriculture and Forestry Research Organisation, 117-124.
Brunt AA. 1995. Genus Carlavirus. In: Murphy F, Fauquet CM, Bishop DHL, Ghabrial SA, Jarvis AW, Martelli GP, Mayo MA, Summers MD, editors. Virus Taxonomy: Classification and Nomenclature of Viruses. Sixth Report of the International Committee on Taxonomy of Viruses. Archives of Virology, Supplement 10. Vienna: Springer-Verlag, 475-478.
Brunt AA, Kenten RH. 1973. Cowpea mild mottle, a newly recognized virus infecting cowpea (Vigna unguiculata) in Ghana. Annals of Applied Biology, 74(1):67-74;
Dubern J, Dollet M. 1981. Groundnut crinkle virus, a new member of the carlavirus group. Phytopathologische Zeitschrift, 101(4):337-347;
Fauquet C, Lamy D, Thouvenel J-C. 1979. Viral diseases of winged bean in the Ivory Coast. FAO Plant Protection Bulletin, 27:81-87.
Fauquet C, Thouvenel J-C. 1987. Plant viruses in the Ivory Coast. Initiations, Documentations, Techniques, No. 46. Paris, France:ORSTOM, 243
Fortuner R, Fauquet C, Lourd M. 1979. Diseases of the winged bean in Ivory Coast. Plant Disease Reporter, 63(3):194-199
Nain PS, Rishi N, Bishnoi SS. 1994. Profile of viral diseases of cowpea (Vigna unguiculata) in northern India. Indian Journal of Virology, 10(2):128-136.
Reddy DVR. 1991. Crop profile. Groundnut viruses and virus diseases: distribution, identification and control. Review of Plant Pathology, 70(9):665-678.
Saleh N, Baliadi Y, Horn NM. 1989. Cowpea mild mottle virus naturally infecting groundnut in Indonesia. Penelitian Palawija, 4:32-35.
Thottappilly G, Rossel HW. 1992. Virus diseases of cowpea in tropical Africa. Tropical Pest Management, 38(4):337-348.
Scientific name
Cowpea golden mosaic virus (CGMV) (genus Bigeminivirus)
Importance
High
Significance
Unquantified severe losses reported in Nigeria.
Symptoms
Symptoms are exhibited on the leaves and on the whole plant.
Leaves: yellowing, chlorosis, distortion, blistering, blotches
Whole plant: stunting.
|
Cowpea golden mosaic virus (photos: IITA) |
Hosts
Cowpea.
Geographic distribution
Nigeria, Kenya, Tanzania, Pakistan
Biology and transmission
The virus is transmitted by whiteflies Bemisia specie
Detection/indexing methods used at IITA
A combination of methods is used for detection and identification. The methods are based on biological properties of the virus.
- Growing out test : Symptomatology . Symptoms used to characterized the viruses
- Visual inspection: take into consideration may exhibit similar symptoms This requires expertise and adequate field experience and confirmation using confirmatory tests
- Transmission tests : using indicator plants: mechanical sap, grafting, vector
- Physical: Electron microscopy, Serology(ELISA and PCR)
Treatment/control
- Host resistance
- Control of white flies using pesticides
- Production in Pest Free Areas
- Production of virus-free seed to supply to breeders.
- Production of seeds for international distribution under certification scheme
- Field inspection during active growth and roguing of diseased plants
Control of whiteflies (Bemisia specie ) using any of the underlisted pesticides as sprays on the field during active growth:
Chemical control
- Act force 100ml to 20lts water or
- Cyper force 100ml to 20lts water or
- Cyper Diforce 100ml to 20lts water
Protocol
- Spray cowpea with any of the above mentionned insecticide at 7-10 days interval beginning from flower bud initiation.
- In case of severe infestation by whiteflies (Bemisia specie) during seedling stage,
- one spray may be needed before flowering. Normally four applications of insecticide are adequate to control the pests.
- Seed treatment using any available pesticide / seed fumigation using phostoxin
Procedures in case of positive test
All lines testing positive are discarded after different confirmatory diagnostic tests have been conducted.
For import:
- For valuable germplasm material , grow imported material under containment, inspection during active growth, rogue plants with symptoms
- Serological testing of symptomless lines
- Harvest lines found free from the virus and release to breeders for research
For export:
- Seeds from symptomless lines harvested from the multiplication plots meant for international distribution are grown in the screen houses.
- The lines are inspected during active growth in collaboration with Plant Quarantine officials. Plants with symptoms are rougued and incinerated. Leaf samples and seeds from symptomless plants are tested serologically by ELISA or PCR
- Only disease free lines are distributed internationally
References and further reading
IITA. 1977. Highlights of 1976 Research, International Research Institute of Tropical Agriculture, Ibadan, Nigeria, 57 pp
Mushtaq Ahmad. 1978. Pl. Dis. Reptr. 62, 224-226
Scientific name
Cowpea yellow mosaic virus (CYMV)
Other scientific name
Cowpea mosaic comovirus
Importance
High
Significance
Yield losses of 80-100% were reported by Singh and Allen
Symptoms
Symptoms differ with different cultivars
Leaf: mosaic, distortion, blistering
Whole plant: systemic infection, green mottling, stunting, dead
|
Cowpea yellow mosaic virus (photo: IITA) |
Hosts
The hosts include: Vigna unguiculata (cowpea), Chenopodium quinoa (quinoa), Crotalaria juncea (sunn hemp), Glycine max (soyabean), Vigna umbellata (Rice- bean).
Geographic distribution
India, Pakistan, Africa ( Kenya, Tanzania, Nigeria, Togo), Surinam
Biology and transmission
Virus is seed borne and seed transmitted.
Sap transmitted
Vector transmitted by beetles Ootheca mutabilis including other species, grasshoppers, and thrips have been reported as vectors of the virus
Detection/indexing methods at IITA
A combination of methods is used for detection and identification. The methods are based on biological properties of the virus.
- Growing out test : Symptomatology . Symptoms used to characterized the viruses
- Visual inspection: take into consideration may exhibit similar symptoms This requires expertise and adequate field experience and confirmation using confirmatory tests
- Transmission tests: using indicator plants: mechanical sap, grafting, vector
-
Physical: Electron microscopy, Serology(ELISA and PCR)
Treatment/control
- Host resistance
- Production in Pest Free Areas
- Production of virus-free seed to supply to breeders.
- Production of seeds for international distribution under certification scheme
- Field inspection during active growth and roguing of diseased plants.
Control of beetles Ootheca mutabilis and the other vectors using any of the underlisted pesticides as sprays on the field during active growth:
Chemical control
- Act force 100ml to 20lts water or
- Cyper force 100ml to 20lts water or
- Cyper Diforce 100ml to 20lts water
Protocol
- Spray cowpea with any of the above mentionned insecticide at 7-10 days interval beginning from flower bud initiation.
- In case of severe infestation by beetles (Ootheca mutabilis) during seedling stage,
- one spray may be needed before flowering. Normally four applications of insecticide are adequate to control the pests.
- Seed treatment using any available pesticide / seed fumigation using phostoxin
Procedures in case of positive test
All lines testing positive are discarded after different confirmatory diagnostic tests have been conducted
For Import:
- For valuable germplasm material , grow imported material under containment, inspection during active growth, rogue plants with symptoms;
- Serological testing of symptomless lines
- Harvest lines found free from the virus and release to breeders for research
For export:
- Seeds from symptomless lines harvested from the multiplication plots meant for international distribution are grown in the screen houses.
- The lines are inspected during active growth in collaboration with Plant Quarantine officials. Plants with symptoms are rougued and incinerated. Leaf samples and seeds from symptomless plants are tested serologically by ELISA or PCR
- Only disease free lines are distributed internationally.
References
Bock KR. 1971. E. Afri. Agric. For. J. 37, 60-62
Witney WK, Gilmer RM. 1974. Ann. Appl. Biol.77, 17-21
Williams RJ. 1977. Trop.Agric. (Trin.). 54, 61-68
Bacteria - cowpea
Contributors to this section: IITA, Nigeria (M. Ayodele, L. Kumar).
|
Contents: |
Cowpea bacterial blight, Bacterial blight, Leaf spot
Scientific name
Xanthomonas axonopodis pv. vignicola (Burkholder 1944) Vauterin et al. 1995
Other scientific names
Xanthomonas phaseoli f.sp. vignicola (Burkholder) Sabet 1959
Xanthomonas campestris pv. vignicola (Burkholder 1944) Dye 1978
Importance
High
Significance
Although yield losses from the fields have been reported, they have not been quantified
Symptoms
Leaves: pin point water soaked spots on the leaves.
Spots coalesce to form orange lesions surrounded by yellow halo. The bacteria infects also the stems, causing cracking, canker and the pods causing water soaked spots.
Hosts
The major hosts of the bacterial blight pathogen are Vigna unguiculata (cowpea), Crotalaria juncea (sunn hemp), Lablab purpureus (hyacinth bean), Phaseolus vulgaris (common bean), Solanum nigrum (black nightshade), Tephrosia purpurea (purple tephrosia), Vigna mungo (black gram).
Geographic distribution
China, India, Turkey, Botswana, Egypt, Nigeria, S Africa, Sudan, Tanzania, Zimbabwe, Puerto Rico, USA.
Biology and transmission
The bacterium is gram- negative rod, single or in pairs motile by one polar flagellum.Colonies on NBY are yellow and circular. Two biotypes have been isolated and identified morphologically from infected cowpea fields in Nigeria. Isolate 1 which produces yellow colonies on NBYis not sensitive to antibiotic lincomycin while isolate 2 which produces dark yellow/light brown colonies on NBY media is sensitive to lincomycin.
The pathogen is seed borne and seed transmitted. Disease development and spread is favoured by rainfall and the bacterium survives in crop residues.
Detection/indexing methods
Agar method and Serology
Agar test using NBY media, selective media and biochemical analysis:
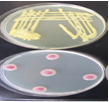
- Randomly select a subsample of 500 seeds (or less if fewer seeds are available) .
- Surface-sterilise the seeds by placing them in a 10% sodium hypochlorite solution for 3 minutes.
- Rinse the seeds with sterile distilled water, blot dry on sterile paper towel, and place seeds equidistantly on NBY agar media in a 9-cm petri dish.
- Incubate seeds at 25oC under 12 h fluorescent light or 12 h NUV light for 4 days.
- Inspect each seed carefully under the stereomicroscope. Use a needle or forceps to turn the seed over and to examine the under side.
- Use the compound microscope for the identification of the bacterium
- Pick bacteria colony and streak unto new NBY plates
- Incubate at 28oC for 48hrs
- On a clean slide, thinly spread bacterial colony; gram stain
- Subculture selected bacteria for further identification on NBY.
- Subject the bacteria to biochemical tests.
- Spot plate the bacterium on MSP and M71 selective media for specie confirmation.
Serology: ELISA using polyclonal antibody for the detection of the bacterium The polyclonal antibody for the detection of X. axonopodis pv vignicola is available in IITA.
Treatment/control
Screen house/ containment facility inspection in collaboration with the National Plant Quarantine service Inspectors for certification and issuance of phytosanitary certificates.
- Seed for export grown in the screen house
- Inspection during active growth of all the multiplication sites
- Use of resistant varieties
- Seeds for international distribution grown under seed certification schemes and Pest Free areas
Procedures in case of positive test
Discard
For import: Grow seeds under containment. Inspect during active growth.Laboratory testing of vegetative parts (leaves and stem ), seeds using agar method and ELISA
Positive lines discard, not acceptable for international distribution in compliance to National Plant Protection Organization requiring additional declaration of freedom from the X. axonopodis pv vignicola or that X. axonopodis pv vignicola is not known to occur in the country of origin or multiplication sites.
References and further reading
Elliot C. 1951. Manual of bacterial plant pathogens. 2d ed Chronica Botanica:Waltham, Mass
Moretti C, Mondjana AM, Zazzerini A, Buonaurio R. 2006. Occurence of leaf spot on cowpea (Vigna unguiculata) caused by Xanthomanas axonopodis pv. vignicola in Mozambique. [online].
Patel PN, Jindal JK. 1970. Indian Phytopath. Soc. Bull. 6, 28-34.
Watkins GM. 1943. Pl. Reptr. 27, 556

Field symptom (photo: IITA) |
yellow and pale yellow colonies (photo: IITA) |

Field symptom (photo: IITA) |
Scientific name
Xanthomonas axonopodis pv. vignae
Other scientific names
Not reported.
Importance
High
Significance
Not reported.
Symptoms
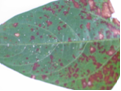
It is a foliar disease. The symptoms are visible on the leaves. On the leaves, the symptoms start as small tiny water soaked dots on the underside of the leaves. The dots coalesce to form circular, raised dark water soaked spots on the underside of the leaves and dark brown necrotic spots on the upper side of the leaves’ Older lesions/ pustules become sunkened and dried in the center.
Leaves turn yellow under severe infection. Defoliation is also a symptom on plants with severe infection. In the field, there is usually some confusion between rust infection (fungal pustules) and bacterial pustules. The first field difference between the two pathogens, is the presence of brown/ pink dust from the leaves of the infected cowpea plants during active growth, this indicates that the pathogen in question is the rust fungi and not a bacterium.
 Bacterial pustule (photo:IITA) |
Hosts
Cowpea.
Geographic distribution
Widely spread in the humid and savannah eco regions where cowpea is grown in Nigeria. The disease has also been reported in Tanzania and Brazil.
Biology and transmission
The pathogen is seed borne and seed transmitted. Disease spread is favoured by rains
Detection/indexing methods used at IITA
Agar method, Serology and leaf isolation:
Agar test using NBY media, selective media and biochemical analysis:
- Randomly select a subsample of 500 seeds (or less if fewer seeds are available) .
- Surface-sterilise the seeds by placing them in a 10% sodium hypochlorite solution for 3 minutes.
- Rinse the seeds with sterile distilled water, blot dry on sterile paper towel, and place seeds equidistantly on NBY agar media in a 9-cm petri dish.
- Incubate seeds at 25oC under 12 h fluorescent light or 12 h NUV light for 4 days.
- Inspect each seed carefully under the stereomicroscope. Use a needle or forceps to turn the seed over and to examine the under side.
- Use the compound microscope for closer for the identification of the bacterium
- Pick bacteria colony and streak unto new NBY plates
- Incubate at 28oC for 48hrs
- On a clean slide, thinly spread bacterial colony; gram stain
- Subculture selected bacteria for further identification on NBY.
- Subject the bacteria to biochemical tests.
- Spot plate the bacterium on MSP and M71 selective media for species confirmation.
Serology:
Conduct ELISA using polyclonal antibodies for the detection of the bacterium.
Treatment/control
- Seed for export grown in the screen house
- Inspection during active growth
- Use of resistant varieties
- Seeds for international distribution grown under seed certification schemes and Pest Free areas.
Procedures in case of positive test
Discard.
At IITA, for import: Grow seeds under containment. Inspect during active growth. Laboratory testing of vegetative parts (leaves and stem ), seeds using agar method and ELISA.
Positive lines discard, not acceptable for international distribution in compliance to National Plant Protection Organization requiring additional declaration of freedom from X. axonopodis pv vignae or X. axonopodis pv vignae not known to occur in the country of origin or multiplication sites.
References
Patel PN. 1978. 3rd Int. Congr. Pl. Path, Munich, August, 1978. p 72 ( abstr.).
Williams RJ. 1975. PANS. 21, 253-267.
Bacterial halo blight, Halo blight (of beans), Grease spot (of beans), Bacterial bean blight
Scientific name
Pseudmonas syringae pv phaseolicola.
Other scientific names
Pseudomonas medicaginis f.sp. phaseolicola (Burkholder) Dowson 1957
Pseudomonas vignae Gardner & Kendrick
Importance
High
Significance
Halo blight is world wide in distribution in the bean growing regions. Walker and Patel, (1964), reported that epidemics have been recorded in some parts of the USA
In some trials with artificial inoculations, losses in seed yield ranging from 2.8-55.4% were obtained (Anon., 1980). Yield losses of 43% in the UK and between 23 and 43% in Michigan were reported by Allen et al. (1998).
Allen et al. (1998) observed crop losses as a result of halo blight in Lesotho, Rwanda and Zimbabwe.
Symptoms
The pathogen infects all the growing stages of the plant: flowering, podding , pre-emergence, and seedling. The pods, growing points, leaves, seeds, stems and whole plant are also infected.
Symptoms found on the various plant parts are :
Leaves: water-soaked spots that later turn red-brown and necrotic. Lesions; abnormal colours; lime-green halo around the necrotic lesion. Pod: water-soaked, greasy spots that vary in size with brown margins. Seeds: rot, shrivelling and discoloured, some times infected seeds are symptomless. Stem: girdling and rotting of nodes, discoloration; and exudates. Whole plant: seedling blight, chlorosis, dieback, lime green coloration, stunting and distorted (Allen et al., 1998).
Hosts
Cowpea (Vigna unguiculata) is considered to be one of the minor host of the bacterium.
The major hosts infected by the bacterium are: Phaseolus acutifolius (tepary bean), Phaseolus coccineus (runner bean), Phaseolus lunatus (lima bean), Phaseolus vulgaris (common bean).
The minor hosts are: Cajanus cajan (pigeon pea), Centrosema , Desmodium (tick clovers), Glycine max (soyabean), Lablab purpureus (hyacinth bean), Pisum sativum (pea), Pueraria montana var. lobata (kudzu), Vigna angularis (adzuki bean),and Vigna radiata (mung bean).
Geographic distribution
Worldwide
Biology and transmission
Non-sporulating, Gram-negative, aerobic rods. Motile by means of multitrichous polar flagellae. Bacterial colonies are white to cream on agar medium with a bluish colour, producing a green fluorescent pigment on King's medium B agar. Optimum growth temperatures for the bacterium are 20-23°C.
Nine races of the halo blight pathogen isolated from Africa and other bean growing areas, have been characterized based on their reactions to eight differential cultivars. (Taylor et al., 1996). Ariyarathne (1997) identified two new races that occurred in Nebraska, USA.
The pathogen can be stored for up to 5 years at -20°C (Schwartz, 1989).
The disease is seed borne and seed transmitted.
The pathogen survives in infected seed and plant residues on the soil surface. Halo blight is favoured by cool, wet weather (Allen et al., 1996). The bacteria multiply rapidly under favourable environmental conditions with or without the formation of lesions. The water-soaking results from extracellular polysaccharides from bacterial slime interacting with plant tissue (El-Banoby and Rudolph, 1979 After penetration, symptoms develop within 6-10 days at 24-28°C. Halo formation is more common at 16-20°C. Lesions can be without halo at temperatures of 28°C and above (Schwartz, 1989). The bacterium produces a toxin, phaseolotoxin, which contains N-phosphosulfamylornithine. This toxin is responsible for the typical halo symptoms and general chlorosis (Schwartz, 1989).
Detection/indexing methods used at IITA
Agar test using NBY media:
- Randomly select a subsample of 500 seeds (or less if fewer seeds are available).
- Surface-sterilise the seeds by placing them in a 10% sodium hypochlorite solution for 3 minutes.
- Rinse the seeds with sterile distilled water, blot dry on sterile paper towel, and place seeds equidistantly on NBY agar media in a 9-cm petri dish.
- Incubate seeds at 25oC under 12 h fluorescent light or 12 h NUV light for 4 days.
- Inspect each seed carefully under the stereomicroscope. Use a needle or forceps to turn the seed over and to examine the under side.
- Use the compound microscope for closer for the identification of the bacterium
- Pick bacteria colony and streak unto new NBY plates
- Incubate at 28oC for 48hrs
- On a clean slide, thinly spread bacterial colony; gram stain
- Subculture selected bacteria for further identification on NBY.
- Subject the bacteria to biochemical tests.
- Spot plate the bacterium on MSP and M71 selective media for specie confirmation.
Treatment/control
- Removal of infected debris after harvest
- Crop rotation using cereals
- Plant disease free, certified healthy seeds and resistant varieties
- Production of seeds under certification scheme and established Pest Free area
- Field inspection during active growth
- Post entry quarantine processing: ELISA , and growing on test under containment to prevent export/ import of infected seeds for research and conservation
Procedures in case of positive test
Discard
For import:
- Post entry quarantine processing by growing the lines under containment facility to intercept the bacteria.
- Inspection during active growth in company of the Plant quarantine inspectors
- Rogue lines with symptoms and incinerate
- ELISA testing for all symptomless lines
- Discard positive lines by incineration
For export:
- Seeds harvested from the multiplication fields are seed health tested
- Using the agar method and selective media previously described in addition to ELISA test. Infect lines are discarded by incineration.
- Not acceptable for international distribution
References and further reading
Allen DJ, Buruchara RA, Smithson JB. 1998. Diseases of common bean. In: Allen DJ, Lenne J, editors. The Pathology of Food and Pasture Legumes. Wallingford, UK: CAB International, 214.
Anon. 1980. Germplasm screening for desirable variability. Disease loss studies. Centro Internacional de Agricultura Tropical: 1979 Bean program. Annual Report.1980, 19-22.
Ariyarathne HM. 1997. Pathogenic variation for the halo blight bacterium and mapping of loci for multiple diseases in common bean. PhD diss. University of Nebraska, Lincoln
El-Banoby FE, Rudolph K. 1979. A polysaccharide from liquid cultures of Pseudomonas phaseolicola which specifically induces water-soaking in bean leaves (Phaseolus vulgaris L.). Phytopathologische Zeitschrift, 95(1):38-50;
Schwartz HF. 1989. Halo blight. In: Schwartz HF, Pastor-Coralles MA, editors. Bean Production problems in the Tropics. Cali, Colombia: Centro Internacional de Agricultura Tropical (CIAT).
Taylor JD, Teverson DM, Allen DJ, Pastor Corrales MA. 1996. Identification and origin of races of Pseudomonas syringae pv. phaseolicola from Africa and other bean growing areas. Plant Pathology
Walker JC, Patel PN. 1964. Splash dispersal and wind as factors in epidemiology of halo blight of bean. Phytopathology, 54:140-141.

Field Symptom (photo: IITA) |
Growth on MSP (photo: IITA) |
Control plate of MSP (photo: IITA) |
Scientific name
Pseudmonas syringae pv syringae
Other scientific names
Phytomonas syringae (van Hall) Bergey et al. 1930
Pseudomonas vignae var. leguminophila (Burkholder) Magrou & Prévot 1948
Importance
High
Significance
P. syringae pv. syringae is seedborne on several crops throughout the world. It attacks several economic crops causing great losses. The bacterium was reported to have caused epidemics on Phaseolus vulgaris cvBonusplantingsin the Transvaal highveld in South Africa where crop losses of up to 55% were reported. The pathogen was also detected in commercial seed stocks (Serfontein, 1994).
Symptoms
-
P. syringae pv. syringae attacks the flowering, podding , post-harvest, seedling and vegetative growing stages of the plant. It also affects the leaves, stems, inflorescence, pods, seeds, roots, and the whole plant
-
Some infected plants are symptomless
-
The bacterium causes frost injury to plants, at relatively high freezing temperatures
-
Symptoms found on the various plant parts are:
-
Leaves : small, water-soaked spots first appearing on the lower sides of the leaves, the spots enlarge, coalesce, form necrotic lesions, blacken and die
-
Stem: water-soaked, sunken brown lesions, splitting at the surface, girdling,
-
Pods: small water-soaked spots , enlarge, coalesce, turn brownish or reddish colored with age (Agrios, 1988; Hall, 1991).
-
Seeds: discoloration, spots, and
-
Seedling: dieback.
 Field symptom (photo:IITA) |
Hosts
The pathogen has several hosts comprising of mono and dicots.
The major hosts of Ps syringae pv syringae are : Vigna unguiculata , (cowpea)Vitis , (grape)Vitis vinifera , (grapevine)Zea mays (maize)Abelmoschus esculentus , (okra)Allium cepa , (onion)Allium porrum , , (leek)Citrus aurantium , (sour orange)Citrus limon , (lemon)Citrus maxima , (pummelo)Citrus medica , (citron)Citrus reticulata , (mandarin)Citrus sinensis , (navel orange)Citrus x paradisi , (grapefruit)Coffea arabica , (arabica coffee)Cucumis sativus , (cucumber)Cucurbita , (pumpkin)Cucurbita maxima , (giant pumpkin)Cyphomandra betacea , (tree tomato)Juglans regia , (walnut)Lablab purpureus , (hyacinth bean)Lactuca sativa , (lettuce)Lycopersicon esculentum , , (tomato)Malus domestica , (apple)Mangifera indica , (mango)Medicago sativa , (lucerne)Musa x paradisiaca , (plantain)Nicotiana tabacum , (tobacco)Oryza sativa , (rice)Panicum , (millets)Panicum miliaceum , (millet)Passiflora edulis , (passionfruit)Pennisetum glaucum , (pearl millet)Pennisetum purpureum , (elephant grass)Persea americana , (avocado)Phaseolus coccineus , (runner bean)Phaseolus lunatus , (lima bean)Phaseolus vulgaris , (common bean)Piper nigrum , (black pepper)Pisum sativum , (pea)Prunus amygdalus , Prunus armeniaca , (apricot)Prunus avium , (sweet cherry)Prunus domestica , (plum)Rosa , (roses)Sorghum bicolor , (sorghum)Sorghum halepense , (Johnson grass)Sorghum sudanense , (Sudan grass)Triticum aestivum , (wheat)Vicia faba , (broad bean)Vicia villosa , Vigna angularis (adzuki bean)
The only recorded minor host is Chenopodium quinoa (quinoa)
Geographic distribution
Worldwide.
Biology and transmission
P. syringae pv. syringae is an aerobic, unicellular Gram-negative rod, motile having one to several polar flagella. The bacterial colonies are circular, milky-white, raised, glistening, translucent, smooth surface, and entire margin. It produces a green fluorescent pigment on King's B medium. The bacteriium produces two lipopeptide toxins, syringomycin and syringopeptin. (Hutchison and Gross, 1997.
This species is represented by strains which are heterogeneous genetically (Gardan et al., 1997). P. syringae pv. syringae comprise of more than 50 distinct pathogens identified as pathovars (Dye et al., 1980; Young et al., 1996). P. syringae pv. syringae is a pathovar originally isolated from lilac but now found infecting several hosts. The pathogen survives on a number of crops and non-crop species, which serve as sources of primary inoculum for infection ( Hall, 1991a, b.). The bacterium is found in the soil, water and on plant surfaces. The bacterium is seed borne and seed transmitted. Spreads through plant parts, rain and wind.
On cowpea, P. syringae pv. syringae, survives in infected seeds and stems. From the seed, it infects the cotyledons, spread to the leaves or enter the vascular system and cause systemic infection resulting in stem and leaf lesions. Disease spread and intensity is favored by rains.
Detection/indexing methods at IITA
Agar method NBY, followed by:
- Selective medium
- and Semi-selective medium
- Serology ( ELISA)
Treatment/control
Seed treatment using mancozeb has not been effective.
- Use of disease-free seeds
- Plant resistant varieties. These are available in IITA
- Crop rotation, discard of plant debris after harvest
- Seed multiplication for international distribution in Pest Free Areas.
Procedures in case of positive test
For import:
- Post entry quarantine processing by growing the lines under containment facility to intercept the bacteria.
- Inspection during active growth in company of the plant quarantine inspectors
- Rogue lines with symptoms and incinerate
- ELISA testing for all symptomless lines
- Discard positive lines by incineration
For export:
- Seeds harvested from the multiplication fields are seed health tested
- Using the agar method and selective media previously described in addition to ELISA test. Infected lines are discarded by incineration.
- Not acceptable for international distribution
References and further reading
Agrios GN. 1988. Plant pathology. London, UK: Academic Press Inc. (London) Ltd.
CAB International. 2007. Crop Protection Compendium, 2007 Edition. Wallingford, UK: CAB International
Goszczynska T, Serfontein JJ. 1998. Milk-Tween agar, a semiselective medium for isolation and differentiation of Pseudomonas syringae pv. syringae, Pseudomonas syringae pv. phaseolicola and Xanthomonas axonopodis pv. phaseoli. Journal of Microbiological Methods, 32(1):65-72.
Hall R. 1991. Compendium of Bean Diseases. St Paul, Minnesota, USA: APS Press.
Higley PM, McGee DC, Burris JS. 1993. Development of methodology for non-destructive assay of bacteria, fungi and viruses in seeds of large-seeded field crops. Seed Science and Technology, 21(2):399-409
Hutchison ML, Gross DC. 1997. Lipopeptide phytotoxins produced by Pseudomonas syringae pv. syringae: comparison of the biosurfactant and ion channel-forming activities of syringopeptin and syringomycin. Molecular Plant-Microbe Interactions, 10(3):347-354
Mohan SK, Schaad NW. 1987. An improved agar plating assay for detecting Pseudomonas syringae pv. syringae and P. s. pv. phaseolicola in contaminated bean seed. Phytopathology, 77(10):1390-1395
Serfontein JJ. 1994. Occurrence of bacterial brown spot of dry beans in the Transvaal province of South Africa. Plant Pathology, 43(3):597-599.
Fungi - cowpea
Contributors to this section: IITA, Nigeria (M. Ayodele, L. Kumar).
Scientific names
Colletotrichum lindemuthianum, Glomerella lindemuthiana Shear [teleomorph]
Other scientific names
Gloeosporium lindemuthianum Sacc., Gloeosporium socium Sacc.
Importance
High
Significance
Anthracnose affects yield, seed quality and marketability of the crop. The disease causes huge losses in temperate and subtropical zones. Losses of 35,925 tonnes due to anthracnose have been estimated in Rwanda. (Tu, 1988). Yield losses of 95% have been recorded in Colombia and over 92% in Malawi (Allen, 1983). In East Africa, anthracnose is important in Kenya, Uganda and Tanzania. It is recurrent in the Great Lakes Region of Rwanda, Burundi and the Kivu Province of Zaire (CIAT, 1981).
In South America, it had been reported that C. lindemuthianum caused severe damage in Brazil (Vieira, 1983), Argentina (Ploper, 1983), Mexico (Crispin-Medina and Campos-Avila, 1976), Guatemala, Costa Rica, Nicaragua (Echandi, 1976), Peru, Ecuador, and Colombia (Olarte et al., 1981.
Symptoms
The fungus infects all stages of the plant, flowering , podding, pre-emergence, seedling and vegetative growing stages and all plant parts including the pods, leaves and seeds. Initial symptoms may appear on cotyledonary leaves as small, dark brown to black lesions.The infected tissues manifest minute rust-coloured specks. The specks gradually enlarge longitudinally and form sunken lesions or eye-spots.
Leaves: lesions first develop on leaf petioles, the lower surface of leaves and leaf veins as small, angular, brick-red to purple spots which become dark brown to black. Later, the lesions may also appear on veinlets on the upper surface of leaves.
Seedlings: lesions enlarge on the hypocotyl of the young seedling, causing rot.
Stem: eye-shaped lesion develop.
Pod: infections appear as rusty brown spots with small, brown specks, sunken cankers delimited by a slightly raised black ring and surrounded by a reddish-brown border. young pods shrivel and dry up.
Seed: discolouration, dark brown to black cankers, brown to light chocolate spots on the seed coats.
 C. lindemuthianum (photo: IITA) |
Hosts
The major hosts infected by this fungus are Vigna unguiculata (cowpea), Cajanus cajan (pigeon pea), Lablab purpureus (hyacinth bean), Phaseolus (beans), Phaseolus vulgaris (common bean), Vigna sinensis ssp. sesquipedalis (asparagus bean.
The minor hosts include Glycine max (soyabean), Lens culinaris ssp. culinaris (lentil), Phaseolus coccineus (runner bean), Pisum sativum (pea), Vicia faba (broad bean), Vigna mungo (black gram), Vigna radiata (mung bean), and Canavalia ensiformis (gotani bean.
Geographic distribution
Worldwide
Biology and transmission
Conidia which are round or elongated are borne on acervuli which may be present on pods, leaves, stems and branches. The mycelium is hyaline, branched and septate.
A conidium takes 6-9 hours to germinate under favourable environmental conditions. The pathogen penetrates the cuticle and epidermis mechanically (Leach, 1923). Following penetration of host cells, when temperatures are favorable, infectious hyphae enlarge and grow between the cell wall and protoplast for 2-4 days without apparent damage to host cells ).
C. lindemuthianum is seed borne and seed transmitted.
C. Lindemuthianum has various strains classified on the basis of host reaction.Two distinct races have been characterized as alpha and beta. Several new races have been identified in Canada, USA, Europe, Brazil and Africa.
Mordue, (1971a,b) reported that the fungus can survive for at least 2 years in seed. The longevity in infected pods and seeds varies considerably depending on environmental conditions. The pathogen was able to survive for at least 5 years on pods and seeds that were air-dried and kept in storage at 4°C or on dry, infected plant materials left in the field in sealed polyethylene envelopes (Tu, 1983).
C. lindemuthianum survives as dormant mycelium within the seed coat, sometimes even within cells of cotyledons, as spores between cotyledons or elsewhere in the seed (Zaumeyer and Meiners, 1975). The fungus survives in the seed as long as the seed remains viable It also survives in infected crop residues.
Infection is favoured by moderate temperatures between 13 and 26°C; (Ferrante and Bisiach, 1976), while Tu and Aylesworth, (1980) reported that infection is favoured by an optimum temparature of 17-24°C.
Humidity of more than 92% or free moisture is required during all stages of conidium germination, incubation and subsequent sporulation; (Tu, 1982).
Zaumeyer and Thomas, (1957) reported that the dissemination and spread of the conidia, and the development of severe anthracnose epidemics is favoured by wind or rain. C. lindemuthianum required about 10 mm of rain to establish infection (Tu, 1981). Conidia spread may be dispersed within the crop by insects, animals and man, especially when foliage is moist (Zaumeyer and Thomas, 1957).
Detection/indexing methods used at IITA
- Agar method. The Nutrient Broth Yeast extract (NBY) agar medium is used for the detection of the pathogen
- According to ISTA randomly select a sub sample of 500 seeds (or less if fewer seeds are available) from the seed lot.
- Surface disinfection of seeds using 10% Sodium hypochlorite for 3 minutes.
- Rinse the seed in sterile distilled water and blot off excess.
- Plate the material on NBY agar medium and incubate at 28oC for 4days.
- Examine plate under stereo microscope.
- Make microscopic slides of fungal fruiting bodies observed in growth.
- Examine under compound microscope to identify the fungal fruiting bodies and spores isolated from the mycelial growth
- Subculture on NBY to obtain pure cultures of the pathogen for pathogenicity tests/ preservation
- Make microscopic slides of the spores and re examine under the Compound microscope for confirmation and purity.
Treatment/cControl
- Seed treatment with mancozeb(Ethylene Bisdithiocarbamate ) 80g a.i./kg of seeds
- Plant pathogen free healthy resistant varieties. IITA has bred several resistant lines
- Production of seeds for export in Certified Pest Free areas (PFA)
- Fungicidal field sprays in the field during active growth
Procedures in case of positive test at IITA
- Seeds from lines testing positive are treated with mancozeb 80g/kg of seeds. The treated seeds are retested after 3 days. If the pathogen is isolated from the treated lines, the lines are rejected. Not for international distribution in compliance to the importing countries’ phytosanitary regulations.
References and further reading
Allen DJ. 1983. The pathology of tropical food legumes: disease resistance in crop improvement. Chichester, UK: John Wiley & Sons
CAB International. 2007. Crop Protection Compendium, 2007 Edition. Wallingford, UK: CAB International
CIAT. 1981. Potential for field beans in eastern Africa: proceedings of a regional workshop held in Lilongwe, Malawi, 9-14 March 1980. CIAT Series 03EB-1. Cali, Colombia: CIAT.
Crispin A, Campos J. 1976. Bean diseases of importance in Mexico in 1975. Plant Disease Reporter, 60(6):534-535.
Echandi E. 1976. Principal fungus diseases of bean (Phaseolus vulgaris) in the American tropics in different ecological zones. Fitopatologia Brasileira, 1(3):171-177
Ferrante GM, Bisiach M. 1976. Comparison of methods for experimental infection of bean with Colletotrichum lindemuthianum. Rivista di Patologia Vegetale, IV, 12(3/4):99-118;
Leach JG. 1923. The parasitism of Colletotrichum lindemuthianum. Minnesota Agricultural Experiment Station Technical Bulletin, 14.
Mordue JEM. 1971a. Colletotrichum lindemuthianum. Descriptions of Pathogenic Fungi and Bacteria Set 32, Sheet No. 316. Wallingford, UK: CAB International.
Mordue JEM. 1971. Glomerella cingulata. CMI Descriptions of Pathogenic Fungi & Bacteria No. 315. Wallingford, UK: CAB International.
Olarte MD, Osorio G, Puerta OD, Isaza L. 1981. Mechanisms for primary infection by anthracnose (Colletotrichum lindemuthianum) on bean (Phaseolus vulgaris) in Eastern Antioch. Fitopatologia Colombiana, 10(1/2):23-28;
Ploper LD. 1983. Bean diseases in Northwest Argentina and their control. Publicación Miscelánea Estación Experimental Agro-Industrial "Obispo Colombres" de Tucumán, No.74:87-103;
Olarte MD, Osorio G, Puerta OD, Isaza L. 1981. Mechanisms for primary infection by anthracnose (Colletotrichum lindemuthianum) on bean (Phaseolus vulgaris) in Eastern Antioch. Fitopatologia Colombiana, 10(1/2):23-28;
Tu JC. 1981. Anthracnose (Colletotrichum lindemuthianum) on white bean (Phaseolus vulgaris L.) in southern Ontario: spread of the disease from an infection focus. Plant Disease, 65(6):477-480
Tu JC. 1982. Effect of temperature on incidence and severity of anthracnose on white bean. Plant Disease, 66(9):781-783;
Tu JC. 1983. Epidemiology of anthracnose caused by Colletotrichum lindemuthianum on white bean (Phaseolus vulgaris) in southern Ontario: survival of the pathogen. Plant Disease, 67(4):402-404
Tu JC. 1988. Control of bean anthracnose caused by the delta and lambda races of Colletotrichum lindemuthianum in Canada. Plant Disease, 72(1):5-8.
Tu JC, Aylesworth JW. 1980. An effective method of screening white (pea) bean seedlings (Phaseolus vulgaris L.) for resistance to Colletotrichum lindemuthianum. Phytopathologische Zeitschrift, 99(2):131-137;
Zaumeyer WJ, Meiners JP. 1975. Disease resistance in beans. Annual Review of Phytopathology, 13:313-334.
Zaumeyer WJ, Thomas HR. 1957. A monographic study of bean diseases and methods for their control. United States Department of Agricultural Technical Bulletin, 868
Cercospora leaf spots, Leaf spot of cowpea
Scientific name
Cercospora canescens
Other scientific name
Cercospora vignicaulis Tehon
Importance
High
Significance
Yield loss due to seed infection has not been quantified
Symptoms
The symptoms are prominent on the leaves alone. However, the fungus has been isolated from infected seeds which are symptomless. Symptoms found on various plant parts are as follows:
Leaves: subcircular to broadly irregular spots having pale tan to grey centre surrounded by dark brown or reddish margin. The spots coalesce to form round lesions which are brown and necrotic with dark, and slightly depressed edges.
Pods: damaged pods, drying up.
Stem: lesions on the stem, and cotyledons
Hosts
Although the disease occurs mainly on cowpeas and on grain legumes, other major and minor hosts have been identified.
The major hosts are Vigna unguiculata (cowpea), Amaranthus (grain amaranth), Glycine max( soybean) , Lablab purpureus (hyacinth bean), Lycopersicon esculentum (tomato), Phaseolus (beans), Ricinus , Vicia (vetch), Vigna (cowpea), Voandzeia subterranea (bambara groundnut).
Other hosts obtained from artificial inoculations are :
Crotalaria juncea (sunn hemp), Psophocarpus tetragonolobus, (winged bean), Vigna angularis (adzuki bean), Vigna mungo (black gram) and Vigna radiata (mung bean).
Geographic distribution
The disease is widespread in warmer subtropical and tropical regions. The fungus has been reported in the Eastern region of USA (Farr et al., 1989); Bangladesh, China, India, Indonesia, Thailand, Africa, Brazil and Samoa.
Biology and transmission
Abundant fruiting bodies on the lower surface of the leaf. The conidia are uniform in colour, pale to medium brown, multiseptate, medium to large size, conidial scar present on the rounded apex, thickened hilum. Most conidia are formed at 28°C, while at 24°C and 32°C less conidia are formed. The presence of light increases the number of conidia (Mulder and Holliday, 1975).
Detection/indexing methods used at IITA
Blotter method.
Procedure
According to ISTA, randomly select a sub sample of 500 seeds (or less if fewer seeds are available) from the seed lot.
- Surface disinfection of seeds using 10% Sodium hypochlorite for 3 minutes.
- Rinse the seed in sterile distilled water and blot off excess.
- Plate the material on blotter and incubate at 28oC for 4days.
- Examine plate under stereo microscope.
- Make microscopic slides of fungal fruiting bodies observed in growth.
- Examine under compound microscope to identify the fungal fruiting bodies and spores
- isolated from the mycelial growth
- Subculture on NBY to obtain pure cultures of the pathogen .
- Make microscopic slides of the spores and re examine under the compound microscope
- for confirmation and purity. Pick single spores and transfer unto V8 agar for sporulation
(The V8 juice agar contains: 3.0 g of calcium carbonate (CaCO3),2.5 g of glucose 20 g of agar powder, 200 ml of V8 juice adjust to 1 litre and autoclave, cool to about 400C. Add 1g of streptomycin powder to prevent bacterial growth).
- Incubate for 2 days at 27oC. Reexamine for purity. For preservation subculture unto ¼ strength PDA slants. Incubation beyond 3 days causes the spores to collapse.
Treatment/control
- Seed treatment with mancozeb ( Ethylene Bisdithiocarbamate ) 80g/kg of seeds
- Plant pathogen free healthy resistant varieties. IITA has bred several resistant lines.
- Production of seeds for export in Certified Pest Free areas(PFA)
- Fungicidal field sprays in the field during active growth
Procedures in case of positive test at IITA
- Seed treatment with mancozeb 80g/kg of seeds. The treated lines to be retested after 3 days of treatment. If the pathogen is isolated from the treated lines, the lines are rejected. Not for international distribution in compliance to the importing countries’ phytosanitary regulations.
References and further reading
CAB International. 2007. Crop Protection Compendium, 2007 Edition. Wallingford, UK: CAB International.
Farr DF, Bills GF, Chamuris GP, Rossman AY. 1989. Fungi on plants and plant products in the United States. St. Paul, Minnesota, USA: APS Press
Mulder JL, Holliday P. 1975. Cercospora canescens. CMI Descriptions of Pathogenic Fungi and Bacteria, No. 462. Wallingford, UK: CAB International.
Field symptom (photo:IITA) |

growth on agar (photo:IITA) |
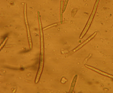
conidia (photo:IITA) |
Scientific name
Cercospora cruenta; Mycosphaerella cruenta Latham [Teleomorph].
Other scientific name
Pseudocercospora cruenta (Sacc.) Deighton
Importance
High
Significance
Fery et al., (1977) reported that M. cruenta reduced the number of pods per plant and the number of seeds per pod. This Cercospora leaf spot disease has been reported by Williams, (1977) to have caused considerable yield losses in cowpea fields in Nigeria.
Schwartz and Pastor-Corrales, (1989) in a study in USA, reported that M. cruenta leaf spot of cowpea reduced the seed yield of the susceptible cv. Colossus by 35.6% . While in Varanasi, India, leaf spot caused by M. cruenta was found to cause serious disease in lobiya (Vigna unguiculata) (Pant, 1989).
Symptoms
The fungus infects the pods, leaves and stems showing various symptoms such as:
Leaves: brown or rust-coloured circular to angular spots, coalesce forming lesions, chlorosis, abnormal leaf fall, and fungal growth on the leaves
Stems: Lesions, discoloration on branches,
Pods: lesions, discoloration
Seed: poor to no germination,small and abnormal
Whole plant: abnormal growth and development.
Hosts
The major hosts for this pathogen are Vigna unguiculata (cowpea), Phaseolus (beans), ) while some recorded minor hosts are Calopogonium, Lablab purpureus (hyacinth bean), Mucuna (velvetbeans), and Mucuna pruriens (Buffalobean).
Geographic distribution
Cosmopolitan
Biology and transmission
The perfect stage produce colourless, 1-septate, ascospores with upper cell which sometimes are slightly larger than the lower cell, straight to slightly curved,
Microconidia are rod-shaped, hyaline, aseptate, produced in pycnidia in or near lesions formed by the imperfect stage, Cercospora. The conidia are thin-walled, filiform, smooth, and hyaline to olivaceous-brown, 4-9-septate. M. cruenta survives between growing seasons on crop residues, diseased leaf and stem crop residues of cowpea (Vigna unguiculata).
The disease is seed borne and seed transmitted
Detection/indexing methods used at IITA
Blotter method.
Procedure
- According to ISTA, randomly select a sub sample of 500 seeds (or less if fewer seeds are available) from the seed lot.
- Surface disinfection of seeds using 10% Sodium hypochlorite for 3 minutes.
- Rinse the seed in sterile distilled water and blot off excess.
- Plate the material on blotter and incubate at 28oC for 4days.
- Examine plate under stereo microscope.
- Make microscopic slides of fungal fruiting bodies observed in growth.
- Examine under compound microscope to identify the fungal fruiting bodies and spores isolated from the mycelial growth.
- Subculture on NBY to obtain pure cultures of the pathogen .
- Make microscopic slides of the spores and re examine under the compound microscope for confirmation and purity. Pick single spores and transfer unto V8 agar for sporulation
- Incubate for 2 days at 27oC. Reexamine for purity. For preservation subculture unto ¼ strength PDA slants
Treatment/control
- Use resistant varieties available in IITA
- Seed treatment with mancozeb( Ethylene Bisdithiocarbamate ) 80g/kg of seeds
- Plant pathogen free healthy resistant varieties.
- Production of seeds for export in Certified Pest Free areas(PFA)
- Fungicidal field sprays in the field during active growth.
Procedures in case of positive test at IITA
- Seed treatment with mancozeb 80g/kg of seeds. The treated lines to be retested after 3 days of treatment. If the pathogen is isolated from the treated lines, the lines are rejected. Not for international distribution in compliance to the importing countries’ phytosanitary regulations.
References
CAB International. 2007. Crop Protection Compendium, 2007 Edition. Wallingford, UK: CAB International
Fery RL, Dukes PD. 1977a. Cercospora leaf spot of southernpea: studies on yield-loss and genetics of resistance. HortScience, 12(3):234.
Pant DC. 1989. Perpetuation of leaf spot organism of lobiya. Indian Phytopathology, 42(1):187-188.
Schwartz HF, Pastor-Corrales MA. 1989. Bean production problems in the tropics, 2nd edition. Cali, Colombia: CIAT
Williams RJ. 1977. Identification of multiple disease resistance in cowpea. Tropical Agriculture, 54(1):53-59.
 |
Scientific name
Fusarium oxysporum f.sp. tracheiphilum (E.F.Sm.) Snyder & H.N. Hansen
Other scientific names
Fusarium bulbigenum var. tracheiphilum, E.F. Sm. Wollenw
Fusarium tracheiphilum E.F. Sm.
Fusarium bulbigenum Cooke & Massee
Importance
High
Significance
Toler et al. (1963) reported that Fusarium wilt of cowpea caused by F. oxysporum f.sp. tracheiphilum is a destructive disease in the Southern and Eastern USA . In India, the yield loss caused by the fungus was 26.8-64.5% in 1954 (Singh, 1954) and 74.6% in 1955 (Singh and Sinha, 1955). And in 1996, the wilt incidence in India was recorded as 30% by Ushamalini (1996).
Symptoms
Fusarium oxysporum f. sp. tracheiphilum attacks young and old plants. Symptoms of the disease are visible on leaves, stem and roots. The vascular bundles show brownish-black discoloration. All plant parts are affected and showing different symptoms such as on:
Leaves: yellowing, withering, Chlorosis, leaf drooping,
Shoots: dry and naked
Stem: blackened and swollen, wilt (Singh and Sinha, 1955
Pods: presence of pinkish-white fungal growth
Seeds: discoloration, shrivelling, ashy white and shrunken (Ushamalini, 1996)
Roots: rot; reduced root system; absence of lateral roots , lesions
Whole plant: systemic infection, colonization of xylem vessels resulting in chlorosis, die back, dwarfing.
Hosts
The major hosts of this pathogen are:
Vigna unguiculata (cowpea) and some minor hosts such as Chrysanthemum vestitum Glycine max (soyabean), and Phaseolus vulgaris (common bean).
Geographic distribution
Cosmopolitan
Biology and transmission
Morphology of F. oxysporum f.sp. tracheiphilum on oat meal agar, has a cotton wool appearance, with a white to purple aerial mycelium. The microconidia are ellipsoidal, hyaline, and aseptate . The macroconidia are slightly curved, mostly septate. The chlamydospores are ellipsoidal to globose, terminal or intercalary and aseptate. The fungus also produces small, abundant sporodochia on PDA in 8-12 days at 26-30°C.The symptoms of vascular wilt are severe at higher temperatures of 27°C (Swanson and Van Gundy, 1985). Swanson, (1984). Harris and Ferris, (1991b) further reported that the wilt symptom is very severe when the fungus is in association with the nematode , Meloidogyne javanica . F. oxysporum f. sp. tracheiphilum is seed borne and seed transmitted. Seeds from wilted plants stored in the refrigerator for 4 years remained viable (Armstrong and Armstrong, 1950). The fungus was found to be present in the seed coat, cotyledon, and embryo.
Detection/indexing methods used at IITA
Agar method
Procedure
- Randomly select a sub sample of 500 seeds (or less if fewer seeds are available) from the seed lot. Surface disinfection of seeds using 10% Sodium hypochlorite for 3 minutes.
- Rince the seed in sterile distilled water and blot off excess.
- Plate the material on NBY agar medium and incubate at 28oC for 4days.
- Examine plate under stereo microscope.
- Make microscopic slides of fungal fruiting bodies observed in growth.
- Examine under compound microscope to identify the fungal fruiting bodies and spores isolated from the mycelial growth
- Subculture on PDA to obtain pure cultures of the pathogen for pathogenicity tests/ preservation
- Make microscopic slides of the spores and re examine under the Compound microscope for confirmation and purity.
Treatment/control
- Use resistant varieties. Available at IITA.
- Seed treatment with mancozeb( Ethylene Bisdithiocarbamate ) 80g/kg of seeds
- Plant pathogen free healthy resistant varieties.
- Production of seeds for export in Certified Pest Free areas(PFA)
- Growing on test in the screen house, active growth inspection, wilted plants rogued and incinerated
Procedures in case of positive test at IITA
- Seed treatment with mancozeb 80g/kg of seeds. The treated lines to be retested after 3 days of treatment. If the pathogen is isolated from the treated lines, the lines are rejected. Not for international distribution in compliance to the importing countries’ phytosanitary regulations.
For import or export:
- For valuable germplasm material to be used for crossing by breeders, growing on test in the containment , active growth inspection, wilted plants rogued and incinerated
- Seeds from symptomless plants harvested and retested before release for use.
References and further reading
Armstrong GM, Armstrong JK. 1950. Biological races of the Fusarium causing wilt of cowpeas and soybeans. Phytopathology, 40:181-193
Harris AR, Ferris H. 1991. Interactions between Fusarium oxysporum f.sp. tracheiphilum and Meloidogyne spp. in Vigna unguiculata. 1. Effects of different inoculum densities on Fusarium wilt. Plant Pathology, 40(3):445-456.
ISTA. 1985. International rules for seed testing. Seed Science and Technology, 13:484-487.
Singh RS, Sinha RP. 1955. Studies on the wilt disease of cowpea in Uttar Pradesh. J. Indian Bot. Soc., 34:375-381.
Swanson TA. 1984. Root-knot nematode and Fusarium wilt diseases of cowpea and soybean. PhD. thesis University of California, Riverside.
Swanson TA, Gundy SDVan. 1985. Influences of temperature and plant age on differentiation of races of Fusarium oxysporum f.sp. tracheiphilum on cowpea. Plant Disease, 69(9):779-781;
Thomason IJ, Erwin DC, Garder MJ. 1959. The relationship of the root-knot nematode, Meloidogyne javanica to Fusarium Wilt of Cowpea. Phytopathology, 49:602-606.
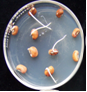
clean seeds (photo:IITA) |

infected seeds (photo:IITA) |
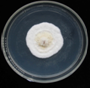
growth on agar (photo:IITA) |
conidia (photo:IITA) |
Scientific name
Sphaceloma sp.
Other scientific names
Elsinoe phaseoli,Jenkins ; Elsinoe vignicola
Importance
High
Significance
High.
Serious disease causing loss of foliage, pods and seeds. In severe case there is complete loss of yield. Exact yield loss caused by this fungus has not been quantified.
The disease is endemic in Zaria, Northern Nigeria.
Symptoms
The fungus attacks the stem, leaves, and pods of Vigna unguiculata
Symptoms on the different parts such as on :
Leaves: spots on both leaf surfaces, cupped. Appearance of small grayish scab lesion along the veins. Leaf distortion, Ragged appearance
Stems: oval to elongated silver grey lesions surrounded by red or brown elliptical ring. Lesions coalesce, distortion
Pod: sunkened spots with grey centers surrounded by brown borders , malformation, dark coloured pycnidia formed in the brown spots.
Hosts
Vigna unguiculata, ( Cowpea) and Lima beans, Beans.
Geographic distribution
The disease has been reported in West and East Africa, Central America and severe outbreaks have been reported in Surinam ( Singh, S R and Allen D. J (1979).
Biology and transmission
The mycelium is hyaline, scanty and submerged. Conidia are hyaline to pale coloured. The conidia are produced in pycnidia. The ascospores borne on the asci are hyaline, pale colored oblong to elliptical, and 3 septate.
Detection/indexing methods used at IITA
- Collect infected plant parts ( stem, leaf, pods and seeds) from the field
- Prepare PDA+streptomycin and rose bengal
- Cowpea Pod Agar amended with PDA adding Streptomycin sulphate (1.5g l-1)
- And Rose Bengal (0.0025g l-1)
- Cut the different infected parts into small portions
- Surface sterilize in 10% sodium hypochlorite for 3 minutes
- Blot with sterile paper towel
- Place five portions in each plate on the medium
- Incubate the plate at 27c for 5-7 days
- Examine under microscope.
Treatment/control
- Use resistant varieties. Available at IITA.
- Seed treatment with mancozeb( Ethylene Bisdithiocarbamate ) 80g/kg of seeds
- Plant pathogen free healthy resistant varieties.
- Production of seeds for export in Certified Pest Free areas(PFA)
Procedures in case of positive test at IITA
- Seed treatment with mancozeb 80g/kg of seeds. The treated lines to be retested after 3 days. If the pathogen is isolated from the treated lines, the lines are rejected. Not for international distribution in compliance to the importing countries’ phytosanitary regulations
- For import or export:
- For valuable germplasm material to be used for crossing, growing on test in the containment , active growth inspection is conducted. Plants with scab symptoms are rogued and incinerated
- Seeds from symptomless plants harvested and retested for freedom from fungus.
References and further reading
Singh SR, Allen DJ. 1979. Cowpea pests and Diseases. IITA monograph. Manual series No 2. Trop. Grain Legume Entomology, IITA, Ibadan, Nigerial.
|
Pod and stem scab (photo:IITA) |
Pod and stem scab (photo:IITA) |
Stem scab (photo:IITA) |
Brown blotch, Brown blotch of cowpea
Scientific name
Colletotrichum truncatum(Schwein.) Andrus & W.D. Moore, 1934
Other scientific names
Colletotrichum dematium f. truncatum, (Schwein.) Arx
Vermicularia truncata Schwein. 1832
Importance
High
Significance
Rheenen, (1975) attributed yield losses of 30% to anthracnose infection in Nigeria. A survey conducted in two states in Brazil detected the disease in 57% of the fields (Lehman et al., 1976). Anthracnose of maturing plants causes serious losses, particularly during the rainy period when shaded lower branches and leaves die due to severe infection.
Symptoms
C.truncatum affects all plant stages and parts - flowering , podding, seedling stages also the pods, inflorescence, leaves, stems and whole plant. The stems, pods and leaves may be infected without showing symptoms. In the advanced stages of anthracnose in the late reproductive stages, infected tissues are covered with black fruiting bodies (conidiomata) which produce setae (minute black spines).
Symptoms found on the different plant parts such as on the:
- Inflorescence: lesions.
- >Leaves: lesions; abnormal colours and forms; fungal growth, necrosis of laminar veins, leaf rolling and petiole cankers. Premature defoliation, girdling of the leaf and petiole Developpment of shepherd's crook .
- Seedlings: discoloration; canker, premature death, and severe reductions in seedling emergence was recorded by Athow, (1987).
- Pods: lesions. irregularly-shaped, brown areas , blanking( no pod formation), reduction in pod size and number.
- Seeds: small, irregular, grey areas with black specks, brown staining, symptomless. The fungus is confined at first to the seed coat. The infected seeds may die during germination or, if they germinate, produce infected seedlings.
- Whole plant: damping off; dwarfing, early senescens.
Hosts
C. truncatum has a wide host range among the local edible legumes. The fungus attacks crops considered as major and minor hosts. The major hosts are Vigna unguiculata (cowpea), Arachis hypogaea (groundnut), Cajanus cajan (pigeon pea), Capsicum annuum (bell pepper), Centrosema , Centrosema pubescens (Centro), Glycine max (soyabean), Medicago sativa (lucerne), Phaseolus (beans), Phaseolus lunatus (lima bean), Phaseolus vulgaris (common bean), Pisum sativum (pea), Vigna (cowpea), Vigna mungo (black gram), Vigna radiata (mung bean).
In addition to the host species mentioned above, C. truncatum has been isolated from several weed species (Hartman et al., 1986; Roy, 1982).
Geographic distribution
Cosmopolitan
Biology and transmission
The hyphae are hyaline, branched and septate
C. truncatum has crowded, black acervuli which are borne on well-developed stromata. It produces numerous, black mixed setae in culture, some are long and others short.
The conidia are borne singly on conidiophores, bluntly tapered, curved, unicellular, and hyaline. Conidia produce one or two short germ tubes which produce dark, sticky appressoria when in contact with the surface of the host plant
The disease is seed borne and seed transmitted .
Detection/indexing methods used at IITA
Seed Health Tests
- Agar method The Nutrient Broth Yeast extract (NBY) agar medium is used for the detection of the pathogen.
Procedure
- Randomly select a sub sample of 500 seeds (or less if fewer seeds are available) from the seed lot.
- Surface disinfection of seeds using 10% Sodium hypochlorite for 3 minutes.
- Rince the seed in sterile distilled water and blot off excess.
- Plate the material on NBY agar medium/ Blotter and incubate at 28oC for 4days.
- Examine plate under stereo microscope.
- Make microscopic slides of fungal fruiting bodies observed in growth.
- Examine under compound microscope to identify the fungal fruiting bodies and spores isolated from the mycelial growth
- Subculture on NBY to obtain pure cultures of the pathogen for pathogenicity / preservation
- Make microscopic slides of the spores and re examine under the compound microscope for confirmation and purity.
Treatment/control
- Seed Treatments with mancozeb followed by 2 foliar sprays of mancozeb.
Procedures in case of positive test at IITA
- Seed treatment with mancozeb 80g/kg of seeds. The treated lines to be retested after 3 days. If the pathogen is isolated from the treated lines, the lines are rejected. Not for international distribution in compliance to the importing countries’ phytosanitary regulations
- For import or export: for valuable germplasm material to be used for crossing, growing on test in the containment , active growth inspection is conducted. Plants with anthracnose symptoms are rogued and incinerated
- Seeds from symptomless plants harvested and retested
References and further reading
Athow KL. 1987. Fungal diseases. In: Wilcox JR, editor. Soybeans: Improvement, Production and Uses, 2nd edition, Monogr. 16. Madison, USA: American Society of Agronomy, 687-727.
Hartman GL, Manandhar JB, Sinclair JB. 1986. Incidence of Colletotrichum spp. on soybeans and weeds in Illinois and pathogenicity of Colletotrichum truncatum.. Plant Disease, 70(8):780-782
Lehman PS, Machado CC, Tarrago MT. 1976. Frequency and severity of soybean diseases in the States of Rio Grande do Sul and Santa Catarina. Fitopatologia Brasileira, 1(3):183-193
Rheenen HA. 1975. Soybeans in the northern states of Nigeria. In: Luse RA, Rachie KO, editors. Proceedings of IITA Collaborators Meeting on Grain Legume Improvement. Ibadan, Nigeria: IITA, 158-159.

Pod symptom (photo:IITA) |
Growth on agar (photo:IITA) |
Conidia and setae (photo:IITA) |
Clean seeds (photo:IITA) |

Infected seeds (photo:IITA) |

Conidia |
Scientific name
Uromyces appendiculatus(Pers.) Unger (1816)
Other scientific names
Uromyces appendiculatus, Uromyces phaseoli,
Uromyces phaseolorum (DC.) de Bary,
Puccinia phaseoli-trilobi Schwein (1834),
Uromyces vignae-luteolae Henn.(1907).
Importance
High
Significance
Infection at the early stages of plant growth , results to crop failure. Yield losses reaching 28-54, 8-33 and 13-29% on different cultivars were reported by Gonzalez and Garcia (1996).
Symptoms
All plant parts above the ground are susceptible to infection. Infection appears at all stages of the plant growth.
- On the Leaves: yellow to yellowish-brown small raised blister like spots, as infection progresses, the blisters erupt and powder like uredo spores are exposed. The spores reinfect the plant and defoliation resulting to death of the plant.
- Stem: brown spots, spores and hyphae are borne internally and externally, spores visible to the eye
- Pods: brown spots, decrease in the number of pods per plant.
- Seed: malformation, decrease in weight of seed , sometimes symptomless
Hosts
The major hosts for the rust fungi are Vigna unguiculata (cowpea), Cajanus cajan (pigeon pea), Glycine max (soyabean), Lablab purpureus (hyacinth bean), Phaseolus (beans), Phaseolus lunatus (lima bean), Phaseolus vulgaris (common bean), Vigna angularis (adzuki bean), Vigna mungo (black gram) and some recorded minor hosts are Phaseolus coccineus (runner bean), Vigna radiata (mung bean), Vigna umbellata (Rice- bean), Voandzeia subterranea (bambara groundnut.
Geographic distribution
Rust is a universal disease of beans and is world wide in spread and distribution.
Biology and transmission
The mycelium is hyaline, branched and septate existing internally in host tissue producing various sori . It is autoecious having white pycnidia in small groups. Aecia are very rare but the aeciospores are globose to ellipsoid. Uredinia , sometimes absent but if present are pale brown, solitary or sometimes aggregated, minute,and cinnamon brown. Urediniospores are globose to subglobose.
Telia are blackish-brown to black. The teliospores are subglobose to ovoid or ellipsoid and rounded at the apex.
There can be crop failure if infection takes place in the early stages of plant growth. Overcast conditions and a temperature of 20-25ºC favour the pathogen. Twenty races of the pathogen were reported by Staveley (1984a) when he used 19 different bean cultivars in an experiment in USA.
Detection/indexing methods used at IITA .
- Two methods are used for isolation of the pathogen. Seed washing method and leaf isolation.
Seed washing method
- Randomly select a sub sample of 500 seeds (or less if fewer seeds are available) from the seed lot and place in a 250-ml flask.
- Add 100 ml of water and 5 drops of tween 80. Shake vigorously for 30 sec.
- Pour 10 ml of the resulting suspension into a test tube and centrifuge in table-top centrifuge for 5 minutes at 4000 rpm.
- Pour off the supernatant and resuspend the pellet in the bit of remaining water by tapping the tube with your finger. Place of a drop of the suspension on a microscope slide, cover with a cover slip, and inspect the entire slide at X10 and above under the compound microscope.
- Look for spores, fruiting bodies. Identify
Leaf isolation method/Slide
- Collect infected leaf sample from the field.
- Harvest Uromyces appendiculatus spores from infected samples using a needle on to a clean slide.
- Add a drop of lactophenol or cotton blue
- View under microscope to check for uredospores
Treatment/control
- Seed treatment with mancozeb followed by field spray before flowering reduced infection but did not eradicate the pathogen
- Plant resistant varieties
- Use disease free seeds and multiply seeds in PFAs
Procedures in case of positive test
- Discard. Not for international distribution in compliance to the importing countries’ phytosanitary regulations
- For import or export: For important germplasm material to be used for crossing, growing on test in the containment , active growth inspection, plants with rust symptoms are rogued and incinerated
- Seeds from symptomless plants harvested and retested
References and further reading
Gonzalez M, Garcia E. 1996. Evaluation of losses due to the rust on bean (Phaseolus vulgaris L.) in four sowing times in Cuba. Agronomia Mesoamericana, 7:95-98.
Stavely JR. 1984. Genetics of resistance to Uromyces phaseoli in a Phaseolus vulgaris line resistant to most races of the pathogen. Phytopathology, 74(3):339-344;
Staveley JR. 1984b. Pathogenic specialization in Uromyces phaseoli in the United States and rust resistance in beans. Plant Disease, 68:95-99.
|
Infected leaf (photo:IITA) |
Infected leaf (photo:IITA) |
Uredospores (photo:IITA) |
Leaf smut, Black spot of pulses
Scientific name
Entyloma vignae
Other scientific name
Protomycopsis phaseoli
Importance
High
Significance
The disease is of economic importance in Brazil where yield losses of 30-40% were reported.
Symptoms
The symptoms are very prominent on the leaves.
Leaves: dark ash grey to black, circular, purplish spots surrounded by yellow hallow border. As infection progresses, spots coalesce, become dark purple leaves become ragged and fall off.
Whole plant: severe infection causes defoliation
Hosts
The only listed hosts are Vigna unguiculata ,(cowpea), Lablab purpureus ,and (hyacinth bean)Vigna radiata (mung bean).
Geographic distribution
India, Africa, Jamaica, Brazil
Biology and transmission
Chlamydospores are black and spherical. Germination by formation of basidia.
Concomitant contamination of seeds.
Detection/indexing methods used at IITA
Seed washing method
- Randomly select a sub sample of 500 seeds (or less if fewer seeds are available) from the seed lot and place in a 250-ml flask.
- Add 100 ml of water and 5 drops of tween 80. Shake vigorously for 30 sec.
- Pour 10 ml of the resulting suspension into a test tube and centrifuge in table-top centrifuge for 5 minutes at 4000 rpm.
- Pour off the supernatant and resuspend the pellet in the bit of remaining water by tapping the tube with your finger. Place of a drop of the suspension on a microscope slide, cover with a cover slip, and inspect the entire slide at X10 and higher under the compound microscope.
- Look for spores, fruiting bodies. Identify
Leaf isolation method/Slide
- Collect infected leaf sample from the field.
- Harvest Entyloma vignaespores from infected leaf or pod samples using a needle on to a clean slide.
- Add a drop of lactophenol or cotton blue
- View under microscope to check for spore identification
Treatment/control
- No effective seed treatment against the pathogen
- Seeds for international distribution are grown in certified PFAs
- Active growth field inspection and certification
- Infected plants rogued
- Seeds not harvested from infected field
Procedures in case of positive test at IITA
- Discard. Not for international distribution in compliance with the importing countries’ phytosanitary regulations
For import or export:
- For important germplasm material to be used for crossing, growing on test in the containment, active growth inspection conducted. Plants with smut symptoms are rogued and incinerated
- Seeds from symptomless plants harvested and retested
|
Leaf infection (photo:IITA) |
Pod infection (photo:IITA) |
Scientific name
Septoria vignae Henn.
Other scientific name
Septoria vignicola Vasant Rao
Importance
High
Significance
Not reported.
Symptoms
Leaf symptoms are very pronounced
Spots coalesce to form dark red circular lesions on both leaf surfaces. Lesions also appear on the pods and stems. Pycnidia are formed in the lesions
 |
 |
|
|
Septoria vignae (photos:IITA) |
||
Hosts
The only major host recorded is Vigna unguiculata (cowpea).
Geographic distribution
India , East Africa, Savannah zones of tropical Africa, Nigeria
Biology and transmission
Fungus produces conidia in pycnidia. Conidia are septate
Seed borne and seed transmitted
Detection/indexing methods used at IITA
- Collect fresh infected samples from the field.
- Surface sterilized with 5% Na0Cl for 2 mins
- Plate infected leaves/ pods/seeds portions on moist filter paper.
- Incubate at 27oC for 24hrs to allow the development of the pycnidiospores.
- Pick the developed pycnidia and plate on PDA infused with lactic acid.
- Incubate the plates at 27oC for 4-6 days.
- Identify under a compound microscope by picking the pycnidia and break it open with a cover slip to identify the spores
Treatment/control
- Seed treatment with mancozeb.
- Seeds for international distribution are grown in certification schemes
- Active growth field inspection.
- Infected plants rogued
Procedures in case of positive test at IITA
- Discard. Not for international distribution in compliance to the importing countries’ phytosanitary regulations
- For import or export: Seeds are treated with mancozeb and retested after 72 hours for presence /absence of pathogen. Infected lines are rejected.
- For important germplasm material to be used for crossing, growing on test in the containment , active growth inspection, plants with Septoria symptoms are rogued and incinerated
Lamptail pod rot of cowpea, Blight of cowpea, Pod rot of cowpea
Scientific name
Choanephora cucurbitarum (Berk. & Ravenel) Thaxt.
Other scientific name
Choanephora americana A. Møller
Importance
High
Significance
The exact data on yield loss are seldom mentionned although in Nigeria. Cowpea losses were estimated at 7-20% by Oladiran (1980).
Symptoms
C. cucurbitarum infects plant tissues that have been damaged either by insects or by physical means. Symptoms vary considerably on different crops
The fungus causes a number of diseases on a range of crops affecting all plant stages: flowering , fruiting , post-harvest, pre-emergence, seedling and vegetative growing and all plant parts; pods, growing points, inflorescence, leaves, seeds, stems and whole plant.
In Nigeria the fungus causes dieback, stem and leaf blight on cowpea.
Visibly field symptoms of Choanephora cucurbitarum on the host tissues have a white hairy appearance resulting from the tall sporangiophores that produce a cluster of brown sporangiola at their tips. On cowpea, symptoms begin as water-soaked lesions at the leaf margins and tips. These lesions became dry and turned olive-green to light brown. Numerous spiny, long sporangiophores developed during dry weather results to total necrosis of the entire plant Turkensteen, 1979; French, 2000.
Symptoms on various plant parts:
Pods: visible mould and whitish spine like mycelium , rot
Growing points: dead heart.
Inflorescence: lesions.
Leaves: lesions; fungal growth; sooty mould; rot; odour.
Seeds: rot.
Stems: external discoloration; canker; dieback; sooty mould.
Whole plant: dieback.
|
Infected pod (photo:IITA) |
Spores |
Hosts
The major hosts attacked by the fungus are: Vigna unguiculata (cowpea), Abelmoschus esculentus (okra), Amaranthus (grain amaranth), Beta vulgaris var. saccharifera (sugarbeet), Brassica oleracea var. botrytis (cauliflower), Cajanus cajan (pigeon pea), Capsicum (peppers), Capsicum annuum (bell pepper), Capsicum frutescens (chilli), Carica papaya (papaw), Citrullus lanatus (watermelon), Cucumis sativus (cucumber), Cucurbita maxima (giant pumpkin), Glycine max (soyabean), Gossypium (cotton), Ipomoea batatas (sweet potato), Manihot esculenta (cassava), Nasturtium officinale (watercress), Phaseolus vulgaris (common bean), Piper nigrum (black pepper), Pisum sativum (pea), Psidium guajava (guava), Psophocarpus tetragonolobus (winged bean), Ricinus communis (castor bean), Sesamum indicum (sesame), Solanum melongena (aubergine), Solanum tuberosum (potato), Sorghum bicolor (sorghum), Spinacia oleracea (spinach), Vigna mungo (black gram), Vigna radiata (mung bean), and Zea mays (maize).
Geographic distribution
The fungus is worldwide in distribution attacking many crops.
Biology and transmission
C. cucurbitarum produces elongate mycelium without septa. The sporangiola, and typical sporangia are sometimes produced but on separate sporangiophores. The zygospores are formed by fusion of two morphologically similar gametes. Zygospores germinate to produce a sporangium containing sporangiospores French, 2000).
C. cucurbitarum has been reported as a pathogen with 48 species belonging to 37 genera within 17 families. It is a weak parasite that grows on predisposed plants whose tissues have been injured mechanically or damaged by insects during feeding (Cuthbert and Fery, 1975) . Mycelium builds up on the affected plant tissues and enzymes are secreted to overcome the resistance of the healthy tissue, which is then invaded (Agrios, 1978). Makambila and Goma, (1993) reported that Sexual spores on Amaranth debris are the main source of inoculum.The fungus produces thick-walled zygospores which can withstand adverse conditions and germinate when temperatures and moisture conditions are favourable for the production of a sporangium containing sporangiospores. These are then disseminated primarily by air currents (Agrios, 1978).
C. cucurbitarum is seedborne on okra in Malaysia (Tai Luang Huan and Musa Bin Jamil, 1975). The seed borne and seed transmitted nature in cowpea has not been established.
Detection/indexing methods used at IITA
- Blotter method.
Procedure
- Randomly select a sub sample of 500 seeds (or less if fewer seeds are available) from the seed lot.
- Surface disinfection of seeds using 10% Sodium hypochlorite for 3 minutes.
- Rinse the seed in sterile distilled water and blot off excess.
- Plate the material on Blotter and incubate at 28oC for 4days.Examine plate under stereo microscope.
- Make microscopic slides of fungal fruiting bodies observed in growth.
- Examine under compound microscope to identify the fungal fruiting bodies and spores isolated from the mycelial growth.
- Subculture on NBY to obtain pure cultures of the pathogen for pathogenicity / preservation.Make microscopic slides of the spores and re examine under the compound microscope for confirmation and purity.
Treatment/control
- Seed treatment with mancozeb
- Seeds for international distribution are grown in certified PFAs
- Active growth field inspection and certification
- Infected plants rogued
- Seeds not harvested from infected field
Procedures in case of positive test at IITA
- Discard. Not for international distribution in compliance to the importing countries’ phytosanitary regulations
- For import or export: Seeds are treated with mancozeb and retested after 72 hours for presence /absence of pathogen. Infected lines are rejected.
- For important germplasm material to be used for crossing, growing on test in the containment , active growth inspection, plants with C. cucurbitarum symptoms are rogued and incinerated
References and further reading
Agrios GN. 1978. Plant pathology. London, UK: Academic Press Inc
CAB International. 2007. Crop Protection Compendium, 2007 Edition. Wallingford, UK.
French ER. 2000. Choanephora blight. In Compendium of Potato Diseases. Second edition. St. Paul, Minnesota, USA: APS Press, in press
Makambila C, Goma JB. 1993. Choanephora cucurbitacearum Curr., a new pathogenic fungus of Amaranthus in Congo. Cahiers Agricultures, 2(3):217-219
Oladiran AO. 1980. Choanephora pod rot of cowpea in southern Nigeria. Tropical Pest Management, 26(4):396-402
Tai Luang Huan, Musa Bin Jamil M. 1975. Seed-borne pathogens in okra fruit rot. MARDI Research Bulletin, 3(2):38-45
Turkensteen LJ. 1979. Choanephora blight of potatoes and other crops grown under tropical conditions in Peru. Netherlands Journal of Plant Pathology, 85(2):85-86
Insects - cowpea
Contributors to this section: IITA, Nigeria (M. Ayodele, L. Kumar).
Scientific name
Aphis craccivora
Other scientific names
Aphis medicaginis auct. nec. Koch, 1854
Aphis leguminosae Theobald, 1915
Pergandeida craccivora Koch
Importance
High
Significance
This Aphid is a major economic pest of cowpea. The aphid while feeding removes sap from the leaves, pods, seeds and other aerial plant parts causing damage to the plant resulting in yield reductions.
From experimental data and results, infestation with A. craccivora caused significant reductions in seed yield. (Ofuya, 1989). In a Chinese study A. craccivora infestation, resulted in a reduction in plant height to 41.9% , green leaf area and delayed production of harvestable pods by 30 days (Chang and Thrower, 1981).
Infestations of A. craccivora on cowpeas caused reduction in growth and losses in yield (Annan et al., 1995). Attia et al., (1986). Bishara et al., (1984), reported that A. craccivora was the most damaging pest of cowpeas in Egypt, particularly early in the growing season.
In addition to loss due to damage caused by the aphid, A. craccivora is known to be an important vector of plant viral disease, transmitting over 30 plant viruses, (Wightman and Wightman, 1994).
The aphid also produces honeydew, a substrate which attracts fungi (Mayeux, 1984).
Symptoms
The aphids attacks all growing stages and parts of the plant : flowering, seedling and vegetative growing , points including the leaves and the plant as a whole.
Leaves: distortion, stunting of leaflets, lesions; abnormal colours; premature defoliation; sooty mould
Pod : shrivelling. (Ofuya, 1995; Bottenberg et al., 1998).
Growing points: rosette
Seed : shrivelling
Whole plant : stunting, deformities and yield reductions. (Annan et al., 1997)
|
Infested cowpea plant (photo:IITA) |
Aphids |
Hosts
The insect feeds on several plants causing damage. Some reported hosts are Vigna unguiculata (cowpea), Arachis hypogaea (groundnut), Cajanus cajan (pigeon pea), Medicago sativa (lucerne), Vigna radiata (mung bean), Capsicum (peppers), Chenopodium quinoa (quinoa), Cicer arietinum (chickpea), Citrus , Gossypium (cotton), Lablab purpureus (hyacinth bean), Lupinus (lupins), Lycopersicon esculentum (tomato), Phaseolus (beans), Phaseolus vulgaris (common bean), Sesamum indicum (sesame), Solanum tuberosum (potato), Theobroma cacao (cocoa), Trifolium (clovers), Vicia faba (broad bean), Vigna catjang , Vigna mungo (black gram).
Geographic distribution
World wide in distribution. It has been reported in Europe, Asia, Africa, Central America, Canada, Mexico, USA, Argentina, Bolivia, Brazil, Chile, Venezuela, Australia, Fiji, New Zealand.
Biology and transmission
Is a shiny black aphid, feeding on the undersurface of the cowpea leaves ,young stems an pods.
Parthenogenetic reproduction occurrs all year round. The aphid is ovoviviparous, The females retain eggs inside their bodies and give birth to small larvae.Young colonies of this small aphids are found on growing points of plants in association with ants. (Soans and Soans, 1971; Patro and Behera, 1991).
Although A. craccivora is polyphagous, it has preference for Leguminosae.
A. craccivora is dispersed by wind. ( dispersal of the winged forms).
Detection/indexing methods used at IITA
Field inspection during active growth and in storage
Dry seed inspection:
- Spread the seed sample on the seed picking tray.
- Inspect visually using hand lens to pick out insects, seeds that are discolored, damaged or infested, shrunken, malformed, etc. and record on the scoring sheet.
- Place the cover on the seed picking tray and turn over to inspect the other side of the seeds.
- Based on both observations, give a score of 0-5 for each category: 0=no incidence; 1=1-20% incidence; 2=21-40% incidence; 3=41-60% incidence; 4=61- 80% incidence; and 5=81-100% incidence.
- Identification using the Stereo microscope/ visual
Treatment
Chemical pesticides used for the control of the aphid and ants on the field during active growth:
Chemical control
- Act force 100ml to 20lts water or
- Cyper force 100ml to 20lts water or
- Cyper Diforce 100ml to 20lts water
Protocol
- Spray cowpea with any of the above mentionned insecticide at 7-10 days interval beginning from flower bud initiation.
- In case of severe aphids /ant infestation during seedling stage, one spray may be needed before flowering. Normally four applications of insecticide are adequate to control the insect pests.
- Seed treatment using any available pesticide / seed fumigation using phostoxin
Cultural
- Use insect resistant varieties
- Multiplication plots and environs should be weed and ants free
Procedures in case of positive test at IITA
- Infested lines are incinerated. Not acceptable for international distribution
References and further reading
Annan IB, Schaefers GA, Tingey WM. 1995. Influence of duration of infestation by cowpea aphid (Aphididae) on growth and yield of resistant and susceptible cowpeas. Crop Protection, 14(7):533-538.
Annan IB, Ampong-Nyarko K, Tingey WM, Schaefers GA. 1997. Interactions of fertilizer, cultivar selection, and infestation by cowpea aphid (Aphididae) on growth and yield of cowpeas. International Journal of Pest Management, 43(4):307-312.
Attia AA, El-Heneidy AH, El-Kady EA. 1986. Studies on the aphid, Aphis craccivora, Koch. (Homoptera: Aphididae) in Egypt. Bulletin de la Société Entomologique d'égypte, No. 66:319-324.
Bishara SI, Fam EZ, Attia AA, El-Hariry MA. 1984. Yield losses of faba bean due to aphid attack. FABIS Newsletter, Faba Bean Information Service, ICARDA, No. 10:16-18.
Bottenberg H, Tamò M, Singh BB. 1998. Occurrence of phytophagous insects on wild Vigna sp. and cultivated cowpea: comparing the relative importance of host-plant resistance and millet intercropping. Agriculture, Ecosystems & Environment, 70(2/3):217-229
Mayeux A. 1984. The groundnut aphid. Biology and control. Oléagineux, 39(8/9):425-434; [
Ofuya TI. 1989. The effect of pod growth stages in cowpea on aphid reproduction and damage by the cowpea aphid, Aphis craccivora (Homoptera: Aphididae). Annals of Applied Biology, 115(3):563-566.
Patro B, Behera MK. 1991. Mutualism between the bean aphids (Aphis craccivora Koch) and ants. Orissa Journal of Agricultural Research, 4(3-4):238.
Soans AB, Soans JS. 1971. Proximity of the colonies of the tending ant species as a factor determining the occurrence of aphids. Journal of the Bombay Natural History Society, 68(3):850-851
Wightman JA, Wightman AS. 1994. An insect, agronomic and sociological survey of groundnut fields in southern Africa. Agriculture, Ecosystems & Environment, 51(3):311-331.
Bean pod borer, Legume pod borer, Cowpea pod borer
Scientific name
Maruca vitrata
Other scientific names
Maruca testulalis Geyer
Crochiphora testulalis Geyer
Importance
High
Significance
Loss in yield not quantified but it is a major cowpea pest in Nigeria .(Odulaja and Oghiakhe 1993).
Symptoms
Maruca feeds on the tender stems, flower buds, flowers, peduncles, pods, and leaves causing damage to all the plant parts. Symptoms found on:
Flowers: round holes,
Leaves: holes
Pods: distortion
Hosts
The insect attacks 39 hosts of which 37 are leguminous as reported by Rathore and Lal (1998). Arodokoun (1996) listed 23 host plants of M. vitrata in Benin Republic. Some recorded hosts of concern to peasant farmers in the tropics include Vigna unguiculata (cowpea), Cajanus, Cajanus cajan (pigeon pea), Canavalia, Canavalia ensiformis (gotani bean), Fabaceae (leguminous plants), Glycine, Lablab purpureus (hyacinth bean), Phaseolus (beans), Phaseolus lunatus (lima bean), Phaseolus vulgaris (common bean) and Pueraria phaseoloides (tropical kudzu).
Geographic distribution
Belgium, Denmark, France, UK, Asia, China, India, Indoneia, Iran, Japa, Korea, Malaysia, Nepal, Sri Lanka, Thailand, Singapore, Vietnam, Africa, Central America, Mexico, USA, S. America, Australia, Fiji, Papua New Guinea.
Biology and transmission
Adults are not active during the day. They are usually found at rest under the lower leaves of the host plant. They live for an average of 6-10 days, each female laying up to 200 eggs. Ke et al. (1985) recorded seven generations per year in China.
Detection/indexing methods used at IITA
Field inspection during active growth and inspection during storage
Dry seed inspection
- Spread the seed sample on the seed picking tray.
- Inspect visually using hand lens to pick out insects, seeds that are discolored, damaged or infested, shrunken, malformed, etc. and record on the scoring sheet.
- Place the cover on the seed picking tray and turn over to inspect the other side of the seeds.
- Based on both observations, give a score of 0-5 for each category: 0=no incidence; 1=1-20% incidence; 2=21-40% incidence; 3=41-60% incidence; 4=61- 80% incidence; and 5=81-100% incidence.
- Identification under the stereomicroscope/ visual
Treatment
- Plant resistant varieties. (IITA has an insect Rearing Unit which rears Maruca sp for research work on Host pest resistance
- Crop rotation
- Biological control, Insect sterility, Use of pheromones/trapping
- Good Agricultural Practice ( Weed free plots)
Chemical control
- Chemical pesticides used for the control of Maruca on the field during active growth:
- Act force 100ml to 20lts water or
- Cyper force 100ml to 20lts water or
- Cyper Diforce 100ml to 20lts water
Procedures in case of positive test at IITA (Discard)
- Infested lines are incinerated. Not acceptable for international distribution
References and further reading
Arodokoun DY. 1996. Importance des plantes-hotes alternatives et des ennemis naturels indigenes dans le controle biologique de Maruca testutalis Geyer (Lepidoptera, Pyralidae), ravageur de Vigna unguiculata Walp. PhD thesis. Quebec, Canada: Universite Laval, 182.
Ke LD, Fang JL, Li ZJ. 1985. Bionomics and control of the legume pod-borer Maruca testulalis Geyer. Acta Entomologica Sinica, 28(1):51-59.
Odulaja A, Oghiakhe S. 1993. A nonlinear model describing yield loss in cowpea (Vigna unguiculata) due to the legume pod borer, Maruca testulalis Geyer (Lepidoptera: Pyralidae). International Journal of Pest Management, 39(1):61-63.
Rathore YS, Lal SS. 1998. Phylogenetic relationship of host plants of Maruca vitrata.
Indian Journal of Pulses Research, 11(2):152-155.
Singh, S R. 1977. Grain legume Entomology, IITA, Ibadan, Nigeria., 55 pp.
|
Adult on cowpea (photo:IITA) |
Larva stage (photo:IITA) |
Pupa stage (photo:IITA) |
Cowpea weevil, Spotted cowpea bruchid, Cowpea seed beetle
Scientific name
Callosobruchus spp.
Other scientific names
Bruchus quadrimaculatus Fabricius 1792
Callosobruchus ornatus (Boheman 1829)
Importance
High
Significance
In Nigeria, yield loss was estimated to 3% of the annual production in 1961/62 . Infestation starts in the pods in the field and are carried over into storage where infestation continues and substantial losses occur (Ojimelukwe et al., 1999). Decrease seed /grain quality.
Symptoms
The insect attacks the fruiting stage, seeds and all stored grains and products.
Pod : eggs cemented to the surface.
Seeds : Round holes.
Hosts
C. maculatus is a major pest of cowpeas, green gram and lentils).
The hosts attacked are Vigna unguiculata (cowpea), Acacia nilotica (gum arabic tree), Cajanus cajan (pigeon pea), Glycine max (soyabean), Phaseolus (beans), stored products (dried stored products), Vigna radiata (mung bean), and Voandzeia subterranea (bambara groundnut).
Geographic distribution
C. maculatus is widely distributed throughout the tropics and sub-tropics. It is found in China, India, Iran, Pakistan, Sri Lanka, Turkey, Africa, Nicaragua, USA, Brazil, Peru, Venezuela, Australia.
Biology and transmission
Adult Callosobruchus maculatus do not feed on stored products. Short lived having a life span of no more than 12 days. Females lay many eggs up to 115 eggs.The beettle lays eggs on maturing cowpea pods.
Detection/indexing methods used at IITA
Field inspection during active growth and inspection of stored seeds for conservation
Dry seed inspection:
- Spread the seed sample on the seed picking tray.
- Inspect visually using hand lens to pick out insects, seeds that are discolored, damaged or infested, shrunken, malformed, etc. and record on the scoring sheet.
- Place the cover on the seed picking tray and turn over to inspect the other side of the seeds.
- Based on both observations, give a score of 0-5 for each category: 0=no incidence; 1=1-20% incidence; 2=21-40% incidence; 3=41-60% incidence; 4=61- 80% incidence; and 5=81-100% incidence.
- Visual and stereomicroscopic Identification
Treatment/cControl
- Chemical pesticides used for the control in the field and under storage.
- Fumigation of the storage facility
- Seed treatment with phostoxin
Cultural
- Host-Plant Resistance. Resistant varieties are available in IITA
- Harvesting at the right time to prevent infestation of pods in the field
- Cold storage at 4oC
Procedures in case of positive test at IITA
- Discard. Infested lines are incinerated. Not acceptable for international distribution
References and further reading
Ojimelukwe PC, Onweluzo JC, Okechukwu E. 1999. Effects of infestation on the nutrient content and physicochemical properties of two cowpea (Vigna unguiculata) varieties. Plant Foods for Human Nutrition, 53(4):321-332.
 |
 |
 |
 |
|
Cowpea weevil, Source Ayodele/Oguntade (IITA) |
|||
Scientific name
Empoasca spp.
Other scientific name
Empoasca dolichi
Importance
High
Significance
Not reported.
Symptoms
Emposca attacks cowpea seedlings causing
Leaves :discoloration of veins and margins, cupping
Whole plant : stunting, pre mature drying of the plant.
 Empoasca dolichi (photos: IITA) |
Hosts
The leaf hoppers attack Vigna unguiculata (cowpea), Arachis hypogaea (groundnut), and Gossypium (cotton).
Geographic distribution
Africa, Nigeria
Biology and transmission
The hopper lays eggs on the underside of the leaves. Adults expentancy life varies from 30-60 days.
Detection/indexing methods used at IITA
Field inspection during active growth
Dry seed inspection
- Spread the seed sample on the seed picking tray.
- Inspect visually using hand lens to pick out insects, seeds that are discolored, damaged or infested, shrunken, malformed, etc. and record on the scoring sheet.
- Place the cover on the seed picking tray and turn over to inspect the other side of the seeds.
- Based on both observations, give a score of 0-5 for each category: 0=no incidence; 1=1-20% incidence; 2=21-40% incidence; 3=41-60% incidence; 4=61- 80% incidence; and 5=81-100% incidence.
- Visual inspection
Treatment/control
Cultural
- Use resistant varieties
- Planting site sanitation
- Field inspection during active growth
- Removal of infested residues after harvest
- Selection of pest free seeds after threshing for storage
- Seed cold storage at 4oC
- Good store hygiene
Chemical control, applying sprays during active growth with either:
- act force 100ml to 20lts water or
- cyper force 100ml to 20lts water or
- cyper Diforce 100ml to 20lts water.
Protocol
- Spray cowpea with any of the above mentionned insecticide at 7-10 days interval beginning from flower bud initiation.
- In case of severe infestation during seedling stage, one spray may be needed before flowering. Normally four applications of insecticide are adequate to control the insect pests.
Procedures in case of positive test at IITA
- Discard. Infested lines are incinerated. Not acceptable for international distribution
References and further reading
Singh SR. 1977. Grain Legume Entomology,IITA, Ibadan, Nigeria,., 55pp
Singh SR. 1977. Tropical Grain Legume Bull.9.1-7
Singh RS, van Emden HF. 1998. Ann.Rev. Entom., 24, Rev, 255-278
Scientific name
Ophiomyia phaseoli
Other scientific names
Melanagromyza phaseoli Vanschuytebroeck, 1951
Agromyza phaseoli Coquillett, 1899
Agromyza destructor Malloch, 1916
Importance
High
Significance
The bean fly is a serious and destructive pest of cow pea and other edible legumes in the tropical and subtropical regions of Asia Talekar (1990.
In Tanzania yield loss which ranges from 30-50% were reported by Wallace, (1939; Walker, (1960). Also, in 1985, the Asian Vegetable Research and Development Center (AVRDC), reported that in Taiwan damage caused by the fly reduced cowpea yield by 32%.
Symptoms
Symptoms caused by the fly are exhibited on various plant parts where they feed and lay eggs,
Leaves : punctures on the upper side, light yellow spots, larval mines turn dark brown, blotchy and drooping, defoliation
Trifoliate leaves : egg holes, silvery, curved stripes of larval mines which are visible on the underside of the leaves , visible tunnels on the upper side of the leaves.
Leaf petiole : swollen
Root : tunnelling , cortex destruction, swollen
Stem : Tunnelling
Whole plant : stunting , Wilting
Hosts
Vigna unguiculata (cowpea) has been reported as a minor host of this fly. Listed major hosts are Fabaceae (leguminous plants), Phaseolus (beans), Phaseolus vulgaris; (common bean), Vigna radiata (mung bean). Minor and wild hosts of the fly have also been reported which include: Cajanus cajan (pigeon pea), Glycine max (soyabean), Phaseolus lunatus (lima bean), Pisum sativum (pea), Psophocarpus tetragonolobus (winged bean), Vigna angularis (adzuki bean), and Vigna mungo (black gram).
Geographic distribution
Bangladesh, China, India, Indonesia, , Iran, Israel, Japan, Jordan, Malaysia, Nepal, Pakistan, Singapore, Saudi Arabia, Sri Lanka, Thailand, Vietnam, Africa, USA, Australia, Fiji, Papua New Guinea
Biology and transmission
The famales of the bean fly are very active on warm clear days seeking young tender leaves for oviposition. Eggs are laid during the morning hours on the upper side of the leaves, often near the midrib close to the petiole.
Burikam (1980) reported that females lay an average of 77 eggs in cowpea. The adult females live for 23-42 days and males for 31-38 days, and if no food is provided they die in 2-3 days ( Raros (1975) .
Detection/indexing methods used
Field inspection during active growth
Dry seed inspection
- Spread the seed sample on the seed picking tray.
- Inspect visually using hand lens to pick out insects, seeds that are discolored, damaged or infested, shrunken, malformed, etc. and record on the scoring sheet.
- Place the cover on the seed picking tray and turn over to inspect the other side of the seeds.
- Based on both observations, give a score of 0-5 for each category: 0=no incidence; 1=1-20% incidence; 2=21-40% incidence; 3=41-60% incidence; 4=61- 80% incidence; and 5=81-100% incidence.
- Visual and stereomicroscopic identification
Treatment/control
Cultural
- Use resistant varieties
- Planting site sanitation
- Field inspection during active growth
- Removal infested residues after harvest
- Selection of pest free seeds after threshing for storage
- Seed cold storage at 4oC
Chemical control
- Seed treatment using carbofuran
- Applying sprays during active growth with either
- Act force 100ml to 20lts water or
- Cyper force 100ml to 20lts water or
- Cyper Diforce 100ml to 20lts water
Protocol
- Spray cowpea with any of the above mentionned insecticide at 7-10 days interval beginning from flower bud initiation.
- In case of severe flies infestation during seedling stage, one spray may be needed before flowering. Normally four applications of insecticide are adequate to control the insect pests.
Procedures in case of positive test at IITA
- Discard. Infested lines are incinerated. Not acceptable for international distribution
References and further reading
AVRDC. 1985. 1983 Progress report. Shanhua, Taiwan: Asian Vegetable Research and Development Center
Burikam I. 1978. Ecological investigation of the bean fly, Ophiomyia phaseoli (Tryon) (Diptera: Agromyzidae) and its natural enemies in Thailand., 71 pp.; [24 fig., unpublished M.Sc. thesis]
Raros ES. 1975. Bionomics of bean fly, Ophiomyia phaseoli (Tryon) (Diptera: Agromyzidae) and its parasites in Hawaii. Ph. D. Thesis. Honolulu, USA: Department of Entomology, University of Hawaii.
Talekar NS. 1990. Agromyzid flies of food legumes in the tropics. New Delhi, India: Wiley Eastern Limited
Walker PT. 1960. Insecticide studies in East African agricultural pests. III. Seed dressing for the control of beanfly, Melanagromyza phaseoli (Coq.) in Tanganyika. Bulletin of Entomological Research, 50:781-793.
Wallace GB. 1939. French bean diseases and bean fly in East Africa. East African Agricultural Journal, 5:170-175.
Flower Thrips, Bean flower thrips, African bean thrips
Scientific name
Megalurothrips sjostedti Trybom, 1908.
Other scientific names
Taeniothrips sjostedti Trybom
Lundathrips inopinatus Bournier, 1979.
Importance
High
Significance
These Thrips are responsible for the total cowpea yield loss in West Africa
Serious infestation causing the loss of flower resulting to complete loss of yield was reported by Childers and Achor, (1995). Alghali (1992) reported that in Nigeria, yield loss of cowpeas was up to 75% when insects attacked during the flower budding and flowering stages.
Symptoms
The Thrips attack the flowering stage infesting the inflorescence and leaves.
Childers and Achor, ( 1995) reported that feeding by M. sjostedti which begins before the flowers open, damages various parts of the cowpea plant especially the flowers. Infestation of the flower and other plant parts results to:
Inflorescence: distortion and discolouration, abortion, reduced pollen production and flower loss
Leaves: Defoliation
Whole plant : death, yield reduction
 Megalurothrips sjostedti(photos:IITA) |
Hosts
Legumes are the main hosts of M. Sjostedti and this include Vigna unguiculata (cowpea), Cajanus cajan (pigeon pea), Phaseolus vulgaris (common bean). Tamo et al. (1993b).They also attack some other plants considered as minor hosts such as Arachis hypogaea (groundnut), and some wild hosts.
Geographic distribution
Reported to be found throughout Sub-Saharan Africa, from the high rainfall areas of West to the semi-arid areas of Kenya and Sudan. Meanwhile in Nigeria, it is associated with the dry savanna regions in which cowpeas are produced.
Biology and transmission
Rapid breeding, laying eggs on leaf petioles, peduncles , inflorescences and pods was reported by Tamo et al., (1993a). Salifu, (1992) reported that development from egg to adult takes about 19 days at 29°C and 58% RH and adults live for about 23 days.
Taylor (1969) reported that the infestation of cowpea plants begin just before flowering. That the adults fligh by day, with the peak of flight activity occurring between noon and 1 pm at a temperature 23-24°C. It had been observed that, both temperature and light intensity influence flight.
Detection/indexing methods used at IITA
Field inspection during active growth.
Dry seed inspection:
- Spread the seed sample on the seed picking tray.
- Inspect visually using hand lens to pick out insects, seeds that are discolored, damaged or infested, shrunken, malformed, etc. and record on the scoring sheet.
- Place the cover on the seed picking tray and turn over to inspect the other side of the seeds.
- Based on both observations, give a score of 0-5 for each category: 0=no incidence; 1=1-20% incidence; 2=21-40% incidence; 3=41-60% incidence; 4=61- 80% incidence; and 5=81-100% incidence.
- Visual and stereomicroscopic identification
Treatment
Cultural
- Use resistant varieties
- Planting site sanitation
- Field inspection during active growth
- Removal of infested residues after harvest
- Selection of pest free seeds after threshing for storage
Chemical control, applying sprays during active growth with any of the pesticides
- Act force 100ml to 20lts water or
- Cyper force 100ml to 20lts water or
- Cyper Diforce 100ml to 20lts water
Protocol
- Spray cowpea with any of the above mentionned insecticide at 7-10 days interval beginning from flower bud initiation.
- In case of severe Thrips infestation during seedling stage, one spray may be needed before flowering. Normally four applications of insecticide are adequate to control the insect pests.
Biological control
- Research on going on the use of parasitoids and entomopathogens.
Procedures in case of positive test at IITA
- Infested lines are incinerated. Not acceptable for international distribution
References and further reading
Alghali AM. 1992. Insecticide application schedules to reduce grain yield losses caused by insects of cowpea in Nigeria. Insect Science and its Application, 13(5):725-730
Childers CC, Achor DS. 1995. Thrips feeding and oviposition injuries to economic plants, subsequent damage and host responses to infestation. In: Parker BL, et al., eds. Thrips Biology and Management. New York, USA: Plenum Press.
Salifu AB. 1992. Some aspects of the biology of the bean flower thrips Megalurothrips sjostedti (Trybom) (Thysanoptera: Thripidae) with reference to economic injury levels on cowpea (Vigna unguiculata (L.) Walp). Revue de Zoologie Africaine, 106(5):451-459.
Tamò M, Baumgärtner J, Arodokoun DY. 1993. The spatio-temporal distribution of Megalurothrips sjostedti (Trybom) (Thysanoptera, Thripidae) life stages on cowpea, and development of sampling plans. Mitteilungen der Schweizerischen Entomologischen Gesellschaft, 66(1-2):15-34.
Tamò M, Baumgärtner J, Delucchi V, Herren HR. 1993. Assessment of key factors responsible for the pest status of the bean flower thrips Megalurothrips sjostedti (Thysanoptera: Thripidae) in West Africa. Bulletin of Entomological Research, 83(2):251-258
Taylor TA. 1969. On the population dynamics and flight activity of Taeniothrips sjostedti (Tryb.) (Thysanoptera:Thripidae) on cowpea. Bulletin of the Entomological Society of Nigeria, 1969:60-71.



 stog-cowpea
stog-cowpea